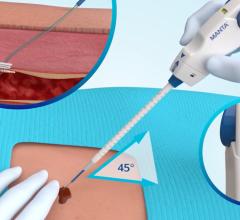
January 12, 2011 – A new, next-generation vascular closure device has been launched in the United States. The Mynx Cadence Vascular Closure Device (VCD), from AccessClosure, offers physicians smoother device deployment while maintaining all the benefits of the original Mynx.
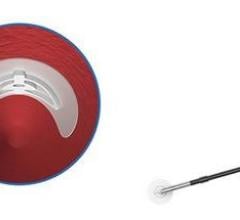
Three design changes on the device make it easier and more consistent to deploy. A definitive shuttle stop when deploying the sealant reduces the possibility of over-shuttling. Additionally, a single marker on the advancer tube removes any guesswork around sealant compression. Finally, a new sealant sleeve protects the sealant during deployment and shortens the procedure time by eliminating the need to pre-soak during device preparation. These changes result in more consistent sealant delivery.
"My initial experience with the Mynx Cadence has been very positive," said Elias Kassab, M.D., FACC, an interventional cardiologist from Oakwood Hospital in Detroit. "The design changes inspire increased confidence in the device, but I don't feel that I've lost any of the tactile feedback I'm used to with the Mynx. These changes truly improve the experience of deploying the Mynx."
It utilizes a conformable, water-soluble polyethylene glycol (PEG) sealant to seal the femoral artery, which dissolves within 30 days, leaving nothing behind but a healed artery. The device received its first FDA approval in May 2007, has been used in over 700,000 procedures and is available in two sizes for 5F and 6F/7F procedural sheaths.
For more information: www.accessclosure.com

 October 07, 2025
October 07, 2025