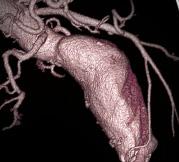
November 20, 2009 – A new service for endovascular specialists who treat aortic aneurysms converts 2D computed tomography (CT) axial images to 3D images of the anatomy and aneurysm measurement for device sizing, precase planning and patient follow-up.
Medtronic announced its partnership this week with Vital Images to create the 3D Recon service. Vital Images is a leading provider of advanced visualization and analysis software. Its
Endovascular Stent Planning application provides real-time automated clinical information specifically for the evaluation of abdominal and thoracic aortic aneurysms. The application enables removal of bone and anatomy from the image, visualization from the aortic root through the iliac bifurcation, as well as specific measurements for procedure planning. It allows vascular surgeons and interventionalists to instantly view 3D anatomy preoperatively, followed by the implanted stent graft post-operatively.
Medtronic said the partnership gives endovascular specialists access to advanced visualization technology on a Medtronic field representative’s laptop to enhance their use of
Medtronic’s aortic stent grafts. 3D Recon complements Medtronic’s existing service offerings of
CTeXpress, a remote DICOM image transfer service in partnership with Intelemage, and Stent
Graft Tracker, software managing patient follow-up schedules.
For more information: www.vitalimages.com, www.medtronic.com


 April 26, 2023
April 26, 2023 









