March 31, 2009 - St. Jude Medical Inc. and GE Healthcare said at ACC that they have commercially launched the first fully integrated wireless solution for the measurement of Fractional Flow Reserve (FFR).
The new FFR solution, which aims to seamlessly integrate into existing cathlab infrastructure, will enable physicians and cath lab staff immediate access to FFR measurement without time consuming setup.
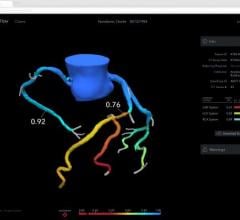
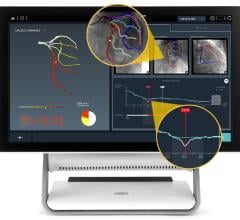
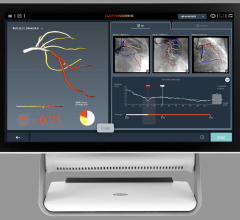
The solution is based on the PressureWire Aeris technology and an FFR upgrade package available for the XT and XTi system configurations of GE’s Mac-Lab hemodynamic recording system, a system used to record and display physiological parameters in the coronary cathlab.
PressureWire Aeris was developed and marketed by Radi Medical Systems, which was acquired in December 2008 by St. Jude Medical and is now part of the its cardiovascular division.
The Mac-Lab FFR upgrade utilizes existing cath lab infrastructure, including screens, input modules and controls, and together with the PressureWire Aeris technology forms a seamlessly integrated FFR measurement system for greatly improved cathlab workflow and ease of use.
“The PressureWire Aeris system represents a true paradigm shift in our thinking about the accessibility of FFR,” said Augusto Pichard, M.D., professor of medicine, cardiology, and director of cardiac cath labs, Washington Hospital Center, Washington, D.C. “The integration with our existing GE hemodynamic recording system means that traditional limitations of FFR measurements, including utilizing a stand alone machine for calculation, are removed.”
With all FFR results integrated into the existing physiological information archive, this new solution is also the only system on the market where the hemodynamic severity of coronary lesions, as measured by FFR, is documented together with other procedural data and angiographic imagery, creating a more complete patient record.
The Mac-Lab FFR solution is available as an upgrade path to all existing GE Mac-Lab installations worldwide, as well as new installations.
For more information: www.sjm.com, www.gehealthcare.com


 February 03, 2026
February 03, 2026