Spacelabs Healthcare debuted Sentinel 11 at the 40th Annual Heart Rhythm Society (HRS)…
Medtronic has received U.S. Food and Drug Administration (FDA) approval for the Attain Stability…
May 6, 2019 — The U.S Food and Drug Administration (FDA) has cleared the Boston Scientific Vici…
May 6, 2019 — The U.S.
Medtronic announced the company has received U.S. Food and Drug Administration (FDA) approval…
Digital healthcare company Murj announced the immediate availability of the Murj 2.0 implantable…
PhaseBio Pharmaceuticals Inc. announced the U.S. Food and Drug Administration (FDA) has granted…
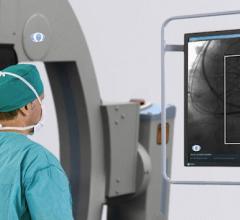
Acutus Medical announced U.S. Food and Drug Administration (FDA) clearance of its second-…
Orchestra BioMed Inc. has secured Breakthrough Device designation by the U.S. Food and Drug…
April 15, 2019 – Intact Vascular Inc. received U.S.
April 15, 2019 — Biotronik began its U.S.
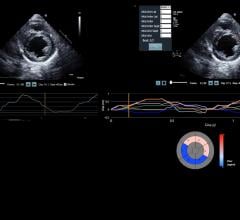
DiA Imaging Analysis announced the launch of LVivo SAX, a cardiac analysis tool that helps…
TherOx Inc. announced that the U.S. Food and Drug Administration (FDA) granted premarket…
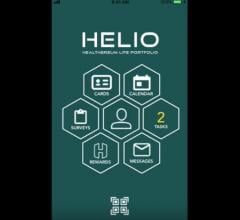
Healthereum LLC released the beta version of the Healthereum Life Portfolio, or HELIO, a mobile…
The U.S. Food and Drug Administration (FDA) granted Omega Medical Imaging 510(k) clearance to…
April 3, 2019 — The U.S.
April 3, 2019 — The U.S.
April 3, 2019 — Essential Medical Inc. received U.S.
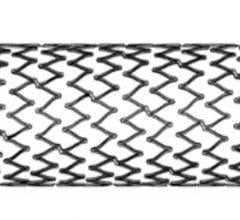
April 3, 2019 — Medtronic's Resolute Integrity Zotarolimus-eluting Coronary Stent System…
Medical imaging and visualization company Medivis announced the launch of AnatomyX, its…


 May 10, 2019
May 10, 2019