Nov. 24, 2025 — InspireMD, Inc., developer of the CGuard Prime carotid stent system for the prevention of stroke ...
Nov. 19, 2025 — Promising results from a pivotal Phase 3 clinical trial of an investigational heart-attack drug were ...
Nov. 18, 2025 — RapidAI announced U.S. Food and Drug Administration (FDA) clearance of Aortic Management, part of the ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Nov. 18, 2025 — Harrison.ai has launched its CE-marked CT Chest solution, a comprehensive AI tool that assists ...
Nov. 11, 2025 -— Integra LifeSciences Holdings Corp. has announced the FDA 510(k) clearance for use of its CUSA Clarity ...
Nov. 17, 2025 — Royal Philips has introduced DeviceGuide, an AI-powered device tracking* solution that assists ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Nov. 17, 2025 — GE HealthCare has announced that Cardiovascular Associates of America (CVAUSA) plans to broaden its ...
Nov. 12, 2025 — The Association of Black Cardiologists (ABC) has released new findings from a national survey exposing a ...
Nov. 13, 2025 — Esaote has announced a new strategic partnership with Schiller Americas. This collaboration strengthens ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Nov. 12. 2025 — The American Heart Association’s Council on Clinical Cardiology has selected Roxana Mehran, MD, as the ...
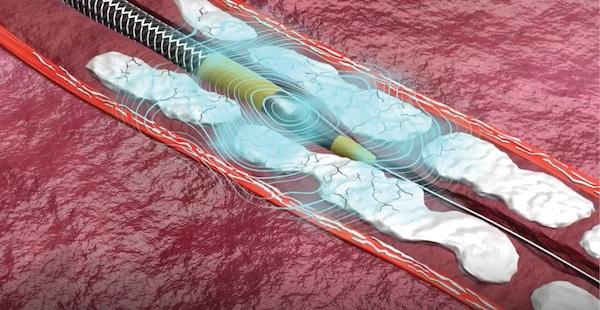
Since receiving FDA approval in 2016, intravascular lithotripsy (IVL) systems have grown in popularity among ...
Nov. 12, 2025 — Siemens has announced plans to deconsolidate its remaining stake in Siemens Healthineers (currently ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Nov. 11, 2025 — FastWave Medical has successfully completed enrollment in its 30-patient coronary feasibility study and ...
Nov. 12, 2025 — On Nov. 11, Huntsman Cancer Institute at the University of Utah (the U) opened its first specialized ...

Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...


 November 24, 2025
November 24, 2025