May 12, 2014 — The Center for Magnetic Resonance Research (CMRR) at the University of Minnesota, Minneapolis, is the first U.S. facility to install Siemens Healthcare’s Magnetom Prisma, a 3T magnetic resonance imaging (MRI) scanner capable of exceptionally high spatial and temporal resolution to achieve image quality, particularly in highly demanding situations.
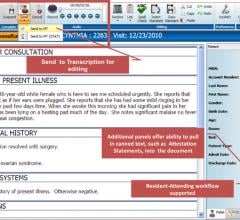
May 12 2014 — M*Modal announced enhancements to its Fluency Flex Mobile dictation application for iOS 6.0+ devices. Moving beyond the capture of clinical notes during patient encounters to more robust clinical documentation, the new application allows physicians to select a patient from their appointment schedule to quickly and accurately record clinical notes, improving ease of use and productivity.
The iCT Elite CT system provides low dose, low energy, and low noise with premium results. It is built on technologies ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
The Ingenuity Elite 256-slice CT scanner uses the NanoPanel Elite for low-dose, low-energy and low-noise imaging. It ...
The Philips Ingenuity Core CT scanner offers low-dose, high-quality imaging and coverage, and the ability to personalize image quality patient by patient. In addition, it offers in-room upgradability so its capabilities can grow as your needs grow. Key benefits of the system include confidence and consistency, high image quality with reduced artifacts, and industry-leading low-contrast resolution. The system comes in both 64 and 128 slice configurations. For more information: www.usa.philips.com/healthcare-products/HCNCTD320/ct-scanners-ingenuity-core-ct-scanner
New data presented for the first time at the World Heart Federation’s World Congress of Cardiology 2014 shows a significant improvement in both patient adherence and risk factor control when patients at high risk of heart attack or stroke receive a polypill, compared to usual care.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
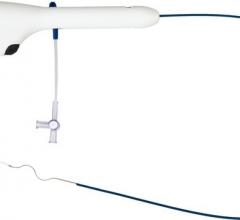
Kalila Medical received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its Vado steerable introducer sheath used during atrial fibrillation (AF) and other procedures requiring vascular and intracardiac access.
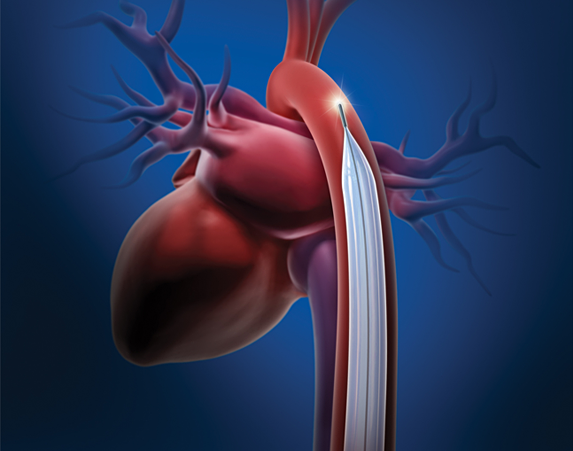
Datascope Corp./Maquet initiated a voluntary worldwide field correction of some of its intra-aortic balloon pumps (IABPs) due to a potential mechanical failure of the fan assembly associated with the power supply. All customers that may have an IABP affected by this field correction have been notified. The company says there are about 12,360 affected units sold globally.

Medical device executives from across the region are not sure the influx of new patients using their devices and the promised sales boost expected from the Affordable Care Act (ACA) will offset the costs of the ACA’s medical device tax, according to a new survey analysis presented to more than 350 medical technology leaders at UMass-Boston at the Massachusetts Medical Device Industry Council’s (MassMEDIC) 17th annual conference.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
InspireMD Inc. initiated a Voluntary Field Action (VFA) following recent reports of MGuard Prime EPS (embolic protection systems), stent dislodgements.
A quarter century ago, doctors treating patients with implanted cardiac pacemakers had a big problem. Their patients were outliving the complex electrical devices that gave them an acceptable quality of life.
May 8, 2014 — Abbott announced its catheter-based MitraClip therapy has received Health Canada approval, providing physicians in Canada with the treatment option that can significantly improve symptoms, disease progression and quality of life for certain people with mitral regurgitation (MR).
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...

May 8, 2014 — The past year has been a turbulent one for U.S. physicians, according to the 2014 Practice Profitability Index (PPI). Approximately 5,064 physicians contributed their insights to the second annual PPI in March 2014.
Argon Medical Devices Inc. began marketing the CelanerXT rotational thrombectomy system as a new addition to the Cleaner family of dialysis products.
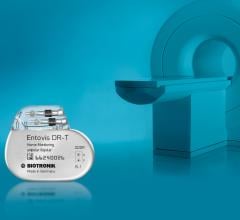
The Food and Drug Administration (FDA) has granted Biotronik approval for its Entovis pacemaker system with ProMRI technology.

 May 12, 2014
May 12, 2014