Invitae Corp., a genetic information company, announced the expansion of its cardiology offering, including more than 30 cardiovascular test panels for arrhythmias, cardiomyopathies, aortopathies, familial hypercholesterolemia, pulmonary hypertension and congenital heart disease.

The Centers for Medicare and Medicaid Services (CMS) released the final version of Stage 3 Meaningful Use (MU) requirements related to healthcare information technology (IT) Oct. 6. CMS will take public comment on the new rules for 60 days beginning Oct. 16.
The results of a research study indicate that interventional cardiologists receive “very high” radiation exposure levels to the left side of the head specifically when performing fluoroscopically guided invasive cardiovascular (CV) procedures. Even with modern imaging equipment and shielding, a significant exposure difference was seen between the two sides of the head.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
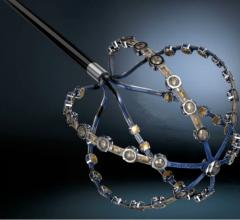
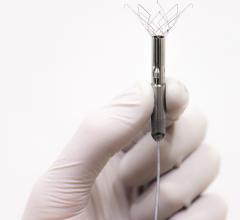
Acutus Medical, a global heart rhythm technology company, presented data that show the AcQMap High Resolution Imaging and Mapping System more rapidly and clearly maps atrial fibrillation (AF) than segmented computed tomography (sCT) mapping, the current standard for most AF ablation procedures. The initial study findings were presented during an oral presentation at the 10th annual Heart Rhythm Congress held in Birmingham, United Kingdom, Oct. 4-7, 2015.
The novel Aortix device has earned a spot among the diagnostic and therapeutic modalities accepted for presentation in the Interventional Innovation “Shark Tank” Competition at the Transcatheter Cardiovascular Therapeutics (TCT) annual scientific symposium. The event will be held Tuesday, Oct. 13, in San Francisco, California.
GE Healthcare announced the creation of a new business unit, Sustainable Healthcare Solutions (SHS), that will develop high-value, low-cost technologies and healthcare delivery solutions across multiple care settings. The new organization will invest $300 million as part of a multi-phase effort to develop a more robust affordable healthcare portfolio for customers. The unit will combine GE Healthcare’s operations in India, South Asia, Africa and Southeast Asia.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
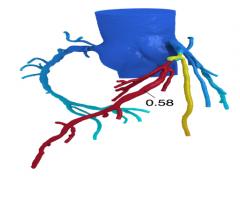
Beaumont Hospital - Royal Oak is the first in Michigan and one of just a handful in the United States to offer fractional flow reserve by computed tomography, also known as FFR-CT. The technology is a noninvasive, diagnostic tool recently approved by the U.S. Food and Drug Administration (FDA) and developed by HeartFlow Inc.
Procyrion Inc. of Houston has been awarded the grand prize of $20,000 in the 2015 "Create the Future" Design Contest for the first catheter-deployed heart pump intended for long-term treatment of chronic heart failure.
October 5, 2015 — The U.S. Food and Drug Administration (FDA) has approved Boston Scientific’s Synergy Bioabsorbable ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Cyberonics Inc. announced results from the extension of the ANTHEM-HF clinical study (ENCORE Study). Results of the study presented by a prominent heart failure (HF) specialist during a major cardiology congress in the United States show that autonomic regulation therapy (ART) in patients with moderate to severe chronic HF and impaired heart function is well-tolerated, safe, improves the heart's ability to pump blood, and reduces the frequency and severity of symptoms associated with chronic HF.
October 2, 2015 — Tryton Medical Inc. announced that results of a post hoc analysis of the pivotal Tryton Randomized ...
AtriCure Inc. launched the cryoFORM cryoablation probe, which offers increased probe flexibility to adapt to a variety of surgical ablation procedures. This offering adds to the cryoICE family of ablation products which are used in the cryosurgical treatment of cardiac arrhythmias.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
AstraZeneca announced that Brilinta (ticagrelor) 60-mg tablets are now available in U.S. pharmacies. On Sept. 3, 2015, the U.S. Food and Drug Administration (FDA) approved a new 60-mg dosage strength for Brilinta to be used in patients with a history of heart attack beyond the first year.
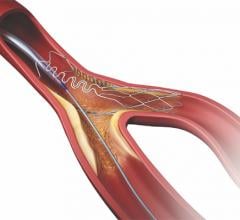
Intact Vascular Inc. announced the U.S. Food and Drug Administration has granted conditional approval for a U.S. and European investigational device exemption (IDE) clinical trial of the Tack Endovascular System.
Kimihiko Kichikawa, M.D., department of radiology at Nara Medical University in Japan, reported two-year results of the Zilver PTX post-market surveillance (PMS) study at the 2015 Cardiovascular and Interventional Radiology Society of Europe (CIRSE) meeting in Lisbon, Portugal. Kichikawa’s presentation focused on initial target data on lesion revascularization (TLR).


 October 08, 2015
October 08, 2015