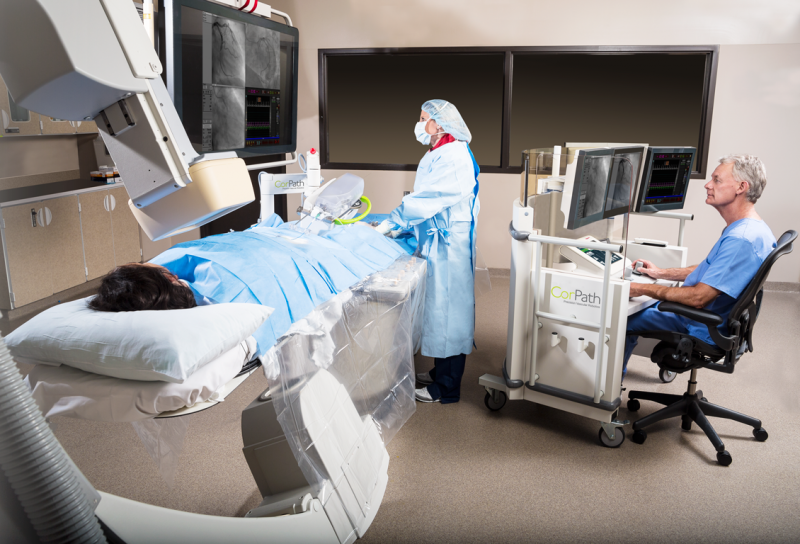
Long-term exposure to ionizing radiation is a primary occupational concern for today's interventional cardiologists. To address these concerns, many interventional cardiologists are enlisting the support of their hospital administrators to create workplace environments that reduce exposure to ionizing radiation.
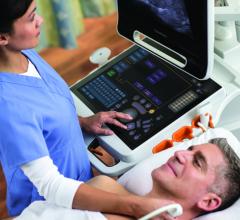
Two all touch-screen interface Carestream Touch Ultrasound Systems have received U.S. Food and Drug Administration (FDA) 510(k) clearance and are now commercially available in the United States. The Touch Prime Ultrasound System and the Touch Prime XE Ultrasound System are both premium systems designed for general diagnostic imaging use in radiology.

Maquet Getinge Group announced the publication of a manuscript describing the exploration of the hemodynamic effects of the newer, larger-capacity 50 cc intra-aortic balloon pumps (IABPs) versus 40 cc IABPs in real-world clinical practice. The paper, titled "Hemodynamic Effects of Standard Versus Larger-Capacity Intraaortic Balloon Counterpulsation Pumps," appears in the April 2015 volume of The Journal of Invasive Cardiology.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Silk Road Medical Inc. announced the company received U.S. Food & Drug Administration (FDA) 510(k) clearance for its Enroute transcarotid neuroprotection system (NPS). The Enroute transcarotid NPS is used to directly access the common carotid artery and initiate high rate temporary blood flow reversal to protect the brain from stroke while performing carotid angioplasty and stenting (CAS).

Mitralign shared six-month data on its Mitralign Percutaneous Annuloplasty System (MPAS) for treatment of functional mitral regurgitation (FMR) at EuroPCR 2015 in Paris. The prospective, multi-center, single-arm study met its safety endpoint at 30 days and its performance endpoint at six months. In the clinical study, the MPAS demonstrated a statistically significant reduction in left ventricular diameter, significant reduction of both the A-P and S-L annular dimensions, and a significant improvement of the patient’s walking distance. The Mitralign Percutaneous Annuloplasty System is not approved for sale or distribution; however it is anticipated to receive CE marking in 2015.
National Security Technologies LLC (NSTec) and Henderson, Nevada-based Global Medical Isotope Systems (GMIS) announced a public-private partnership agreement for research and development of an essential radioactive isotope used in millions of medical diagnostic imaging procedures annually.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Agfa HealthCare announced the launch of its Enterprise Imaging Exchange Program, which establishes a secure health information exchange network between multispecialty collaborating health providers to exchange and share medical imaging data. The solution will be on display at the Society for Imaging Informatics in Medicine (SIIM) annual meeting, May 26-31, 2015, in Washington, D.C.
To meet both clinical and research needs of magnetic resonance (MR) customers, Toshiba America Medical Systems Inc. has implemented software and hardware upgrades for the Vantage Titan 3T MR imaging system. Available for new systems and for existing installations, these upgrades improve image quality and workflow so those in both the clinical and research settings have access to the highest levels of performance and information to provide the best possible care.
The Healthcare Information and Management Systems Society (HIMSS) submitted comments to the Department of Health and Human Services on the Meaningful Use Stage 3 proposed rule and the 2015 Edition Health IT Certification Criteria.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Oxford Instruments plc has acquired Medical Imaging Resources Inc. (MIR). MIR specializes in the build, lease and service of mobile medical imaging labs.
CVRx Inc. announced that positive results from the 'Barostim Therapy for Heart Failure' randomized, controlled clinical trial were presented at the ESC-Heart Failure 2015 Annual Conference in a late-breaking trial session. Results were presented by Prof. Jochen Müller-Ehmsen, M.D., Ph.D., from Asklepios Hospital Altona in Hamburg, Germany.
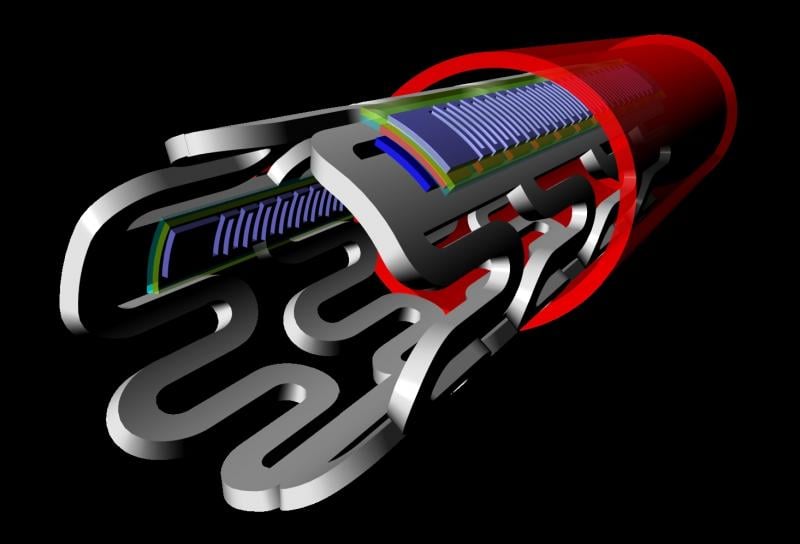
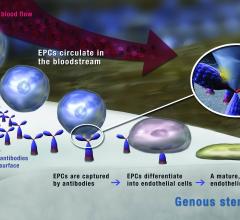
With the recent innovation of flexible microelectronic sensor circuits that can be made from bioresorbable materials, it is now possible to create “electronic stents” to monitor a treated lesion site inside a patient’s artery. A Korean-led team recently published their research on the first device of this kind, showing proof of concept in the American Chemical Society journal ACS Nano.[1]
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
The U.S. Food and Drug Administration (FDA) has cleared the InvisionECG technology from InvisionHeart. The mobile electrocardiogram (ECG) technology uses a secure cloud-based, healthcare IT platform to capture and manage 12-lead ECGs.

A virtual-heart platform proposed by Stony Brook University researchers and colleagues has received funding from the National Science Foundation (NSF) in the amount of $4.2 million over five years. The platform was designed to improve and accelerate medical-device development and testing.
OrbusNeich has announced that the first U.S. patient has been enrolled in the HARMONEE (Harmonized Assessment by Randomized, Multi-center Study of OrbusNEich's COMBO StEnt) stent study. The study is being conducted under the framework of the joint Japan-U.S. Harmonization-By-Doing (HBD) initiative and will support the company's planned application for Shonin approval in Japan and to meet the feasibility trial requirements in the U.S.

 June 02, 2015
June 02, 2015