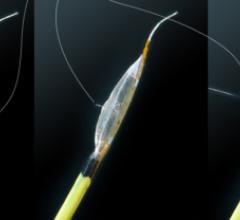
Boston Scientific Corporation has begun the launch of its 0.035-inch and 0.018-inch Rubicon Support Catheter in the United States. The device is designed to assist physicians with placement and support of guidewires that are used in peripheral vascular procedures to deliver stents and balloons to open blockages in the legs and other peripheral arteries. Boston Scientific is also launching the 0.035-inch Rubicon Support Catheter in Europe to build on the momentum of the 0.018-inch size that was introduced in that region last fall.

March 7, 2013 — Ultrasound technology could soon experience a significant upgrade that would enable it to produce high-quality, high-resolution images, thanks to the development of a new key material by a team of researchers that includes a professor in the department of biomedical engineering at Texas A&M University.
March 7, 2013 — A newly identified genetic variant doubles the risk of calcium buildup in the heart’s aortic valve. Calcium buildup is the most common cause of aortic stenosis, a narrowing of the aortic valve that can lead to heart failure, stroke and sudden cardiac death.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
March 7, 2013 — Experts from The Children's Hospital of Philadelphia were among the leaders of two large national studies showing that extending cardiopulmonary resuscitation (CPR) longer than previously thought useful saves lives in both children and adults. The research teams analyzed the impact of CPR duration in patients who suffered cardiac arrest while hospitalized.
MobileDemand and VectraCor Inc. have partnered to provide a turnkey solution to the healthcare industry for patients presenting with chest pain to detect real-time ECG changes suggestive of a heart attack. VectraCor has chosen the xTablet C1200 rugged convertible to use as their mobile, rugged version of the VectraplexECG System.

The American Society of Echocardiography (ASE) has released a list of five interventions whose appropriateness physicians and patients should discuss as part of Choosing Wisely, an initiative of the ABIM Foundation, along with Consumer Reports. First in the list, they ask that patients and their doctors talk about the real need for additional echocardiograms when there are only minor signs of heart valve leakage.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
March 7, 2013 — Philips Healthcare announced it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its EchoNavigator live image-guidance tool.
CardioNet Inc. announced a multi-year development agreement with the Belgium-based nanoelectronics research center IMEC and its Dutch affiliate Holst Centre. Over the next 18 months, the companies will work to develop two revolutionary cardiac monitoring products.
Mercator MedSystems Inc. announced it has received CE mark approval to market its Cricket and Bullfrog Micro-Infusion Catheters in Europe, significantly expanding the market for these products, which have previously received 510(k) clearance from the U.S. Food and Drug Administration (FDA). The Mercator micro-infusion catheters are capable of non-systemic delivery of therapeutic agents directly across any peripheral or coronary blood vessel. Initially, these products will be commercialized for the treatment of peripheral artery disease (PAD), a critical health issue affecting 12-14 percent of the general population and nearly 20 percent of those over the age of 70. Approximately 23 million Western Europeans and 17 million Americans have PAD.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
March 7, 2013 — Cardiovascular Systems Inc. (CSI) presented three-year data from its ORBIT I feasibility study of calcified coronary lesions during a poster session at the 2013 Cardiovascular Research Technologies (CRT) conference.

As the U.S. government works to strengthen its global nuclear security strategy by eliminating potential sources of bomb-grade highly enriched uranium (HEU), it has eyed the production of medical isotopes for nuclear imaging, which rely on HEU. To encourage reliable supplies of medical radioisotopes produced from non-HEU sources, President Obama signed into law on Jan. 2, 2013, the American Medical Isotopes Production Act of 2011 (AMIPA). It should be noted that it was part of the 2013 National Defense Authorization Act, so the measure goes beyond interest in just making sure patients have access to radiopharamceutical tracers to enable myocardial perfusion imaging (MPI) scans. The effort is designed to make HEU sources less available on the world market to reduce the threat of nuclear weapons proliferation or the building of a “dirty bomb.”
In the past few years, especially since the introduction of the iPhone and iPad, there has been an explosion of healthcare related mobile device applications (apps). Most physicians today have smart phones or tablets and are finding ways to integrate them into their daily practice beyond just checking their e-mails.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Brigham and Women’s Hospital in Boston conducted a pilot study of a Web-enabled computerized physician order entry (CPOE) system (another Stage 2 requirement) with embedded imaging decision support. In an effort to reduce the inappropriate use of medical imaging and improve quality of care, it was phased into clinical use between 2000 and 2010 across outpatient, emergency and inpatient departments. The study, published in the Journal of the American College of Radiology in February 2012, showed significant increases in meaningful use for electronically created studies (from 0.4 percent to 61.9 percent) and for electronically signed studies (from 0.4 percent to 92.2 percent) and the adoption of CPOE (from 0.5 percent to 94.6 percent).

In an era of healthcare reform and a push to meet appropriate use guidelines for tests, imaging and therapy amid declining reimbursements, there has been much discussion about implementation of clinical decision support software (CDS). There is apprehension by some physicians who view CDS as technology telling doctors how to practice medicine. There are others in healthcare who are concerned about adding cost with the implementation of this software and how it will be updated based on the most current clinical data and practice guidelines. However, if implemented in a way where it is integrated with workflow and accepted by the physicians and hospital leadership, CDS has helped some hospitals meet appropriate use criteria and reduce unnecessary tests, which in turn helped reduce healthcare costs.
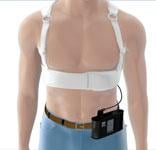
Oklahoma Heart Hospital (OHH), Oklahoma’s first dedicated heart hospital, has excelled at creating quality outcomes through an integrated approach to patient care. To maintain this culture of quality, its team of more than 60 physicians, including interventional cardiologists, general cardiologists, cardiothoracic surgeons and electrophysiologists, practice a systematic process of patient care that includes high adherence to a set of core measures or indicators. Specifically, managing a patient’s risk for sudden cardiac death (SCD) is a critical piece of the care.

 March 08, 2013
March 08, 2013