Cour Pharmaceutical Development Co. Inc. published data in Science Translational Medicine showing the potential of Immune Modifying Nanoparticles (IMP) to reduce inflammation and promote tissue repair and regeneration in patients who have suffered a heart attack.
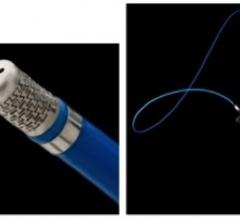
The U.S. Food and Drug Administration (FDA) cleared Radius Medical LLC’s Prodigy Support Catheter. Engineered to provide back-up support to guidewires, the Prodigy Support Catheter is intended for use in treating chronic total occlusions.
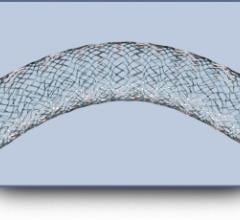
Patients in the European Union (EU) received the first Veniti Vici Venous Stent System implants to treat symptomatic venous outflow obstruction of the lower extremities.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Men with enlarged prostates can find relief with a non-surgical treatment that shrinks the gland, suggests research done on more than 100 patients and presented at the 26th annual International Symposium on Endovascular Therapy (ISET).
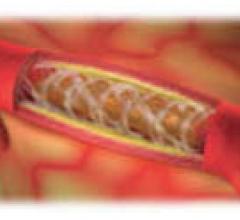
Six-month results of the ESPRIT trial suggest a bioresorbable drug-eluting scaffold is effective in opening blocked blood vessels in the legs and pelvis, as presented at the 26th annual International Symposium on Endovascular Therapy (ISET).
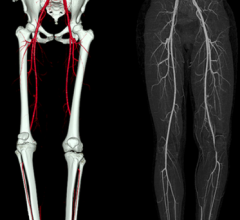
Peripheral artery disease (PAD) sufferers maintain improved quality of life, including being able to walk farther, three years after being treated with stents to open their blocked leg arteries, according to STROLL trial results presented at the 26th annual International Symposium on Endovascular Therapy (ISET).
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...

The U.S. Food and Drug Administration (FDA) has approved many cardiac implantable electronic device models currently in use through a review process in which models were deemed safe and effective based on approval of prior versions of the device, according to a study published in JAMA.
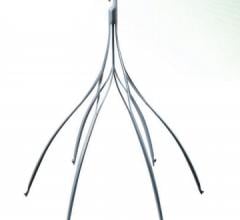
Argon Medical Devices Inc. received clearance from the U.S. Food and Drug Administration (FDA) to begin marketing the OptionElite retrievable inferior vena cava (IVC) filter with an over-the-wire delivery technique.
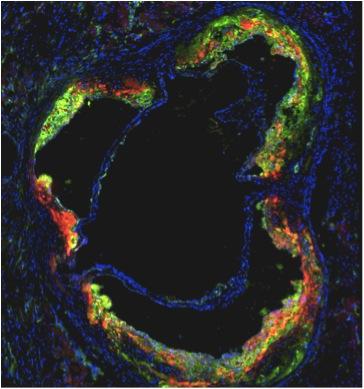
An international research team, led by Icahn School of Medicine at Mount Sinai investigators, designed and tested a high-density lipoprotein (HDL) nanoparticle loaded with a statin drug. In mouse studies, they show HDL nanotherapy is capable of directly targeting and lowering dangerous inflammation in blood vessels.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
An international research team at Icahn School of Medicine at Mount Sinai is testing a sugar-based tracer contrast agent to be used with positron emission tomography (PET) imaging to help identify dangerous inflammation and high-risk vulnerable atherosclerotic plaque inside vessel walls that causes acute heart attacks and strokes.
An implant of the ReZolve2 bioresorbable scaffold was transmitted live via satellite at the Transcatheter Cardiovascular Therapeutics (TCT) 2013 conference in San Francisco.
The three most common systems to place stents in blocked carotid arteries of the neck have similarly low rates of complication and death among U.S. patients, according to a study published by JACC: Cardiovascular Interventions.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Research published in the American Journal of Managed Care indicated using telemedicine to deliver stroke care, or telestroke, appears to be cost-effective for society. Telestroke robots allow stroke patients in facilities with no neurology specialists to receive real-time consultation from neurologists in other areas via computer.
The U.S. Food and Drug Administration (FDA) gave market clearance to Irvine Biomedical Inc., a St. Jude Medical company, for its Therapy Cool Flex Ablation Catheter and IBI 1500T9-CP v.1.7 Cardiac Ablation Generator.
The U.S. Food and Drug Administration (FDA)’s Cardiovascular and Renal Drugs Advisory Committee recommended approval of vorapaxar, Merck’s investigational antiplatelet medicine for the reduction of atherothrombotic events, when added to standard of care in patients with a history of heart attack and no history of stroke or transient ischemic attack.

 January 24, 2014
January 24, 2014