July 6, 2010 – The development of a new magnetic resonance imaging (MRI) technology, which may revolutionize the way medical conditions are diagnosed and treated, is to take a major step forward as a $10.6 million research center is established at the University of York.
July 6, 2010 – The government’s certification program for health information technology (HIT) will continue to evolve over time, according to a review of the recently released final rule for the temporary certification program, released last week.
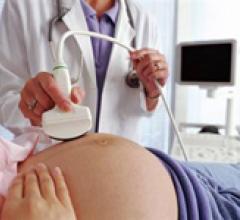
July 6, 2010 – Ultrasound company SonoSite Inc. announced last week that it has completed its acquisition of privately held VisualSonics, a Toronto-based company focused on ultra high-frequency micro-ultrasound technology, for $67.9 million net of cash and debt.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
July 1, 2010 – Two medical companies say they will work with physicians to further investigate ways to manage genetic issues in antiplatelet therapy, after the U.S. Food and Drug Administration (FDA) recently released a warning about the efficacy of the antiplatelet drug clopidogrel (Plavix) on patients with certain genetic make-ups.
July 1, 2010 – The U.S. Food and Drug Administration (FDA) recently added a warning to the information on clopidogrel (Plavix), as a result of new information indicating that variations in genetic makeup can prevent the medication from reducing patients’ risk for heart attack and stroke.
July 1, 2010 – Clinical results have shown the Rheos System is successful in lowering blood pressure, CVRx Inc. announced this week at the European Society of Hypertension Meeting. The system is the first device designed to treat hypertension (high blood pressure) and is a future treatment option for the millions of people who cannot control their blood pressure with medications.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
June 30, 2010 – Thirty percent of cardiology information technology (IT) users are considering replacing their cardiovascular information system (CVIS) software because of ongoing frustrations with product functionality and integration, according to a new KLAS report released this week.
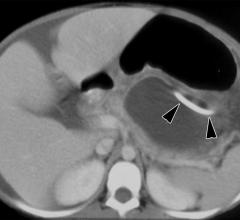
June 29, 2010 – A newly formed committee of experts has begun to tackle the issue of reducing radiation in computed tomography (CT). The panel was launched by Siemens Healthcare as a part of the Siemens Radiation Reduction Alliance (SIERRA).
June 28, 2010 – A new 3-D imaging technology is now available in the U.S. The C-InSight from Mazor Surgical Technologies Ltd. will enable medical facilities to incorporate 3-D imaging capabilities into their existing 2-D C-arms, the standard X-ray device used in operating rooms.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
June 28, 2010 – There is a growing consensus that radiologists must ensure that patients undergoing computed tomography (CT) receive the minimum radiation dose possible to produce a medical benefit, according to an article in the June 23 issue of the New England Journal of Medicine.
June 28, 2010 – Saint Francis Health System announced last week that it has chosen a new cardiology information system. Wolters Kluwer Health will provide its ProVation MD software for comprehensive cardiology procedure documentation and coding at Saint Francis Hospital.
June 28, 2010 – AGA Medical has been denied the right to appeal a decision in a United Kingdom patent case concerning structural heart occluders.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
June 24, 2010 – Tryton Medical Inc. announced that the company’s Tryton Side Branch Stent System has been used in three cases in Israel for the first time. Yaron Almagor, M.D., director of the interventional cardiology and cardiac catheterization laboratories at Shaare Zedek Medical Center in Jerusalem, Israel, performed the first implants.
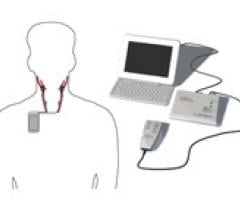
June 24, 2010 – A new recorder upgrade will provide extended recording time for physicians, increasing their detection of abnormal heart rhythms in patients that could otherwise be missed. Midmark Corp. this week announced the release of an upgrade kit providing 48- and 72-hour recording capabilities for its IQholter recorders.
June 24, 2010 – The U.S. Food and Drug Administration (FDA) has cleared a new catheter and software for treating cardiac arrythmias. Biosense Webster Inc. announced yesterday that the FDA has cleared for marketing the CartoXPress Software Module and the Lasso NAV Circular Mapping Catheter for use with the Carto XP Mapping System.

 July 06, 2010
July 06, 2010