August 12, 2014 — Like clues to a crime, specific molecules in the body can hint at exposure to toxins, infectious agents or even trauma, and therefore help doctors determine whether and how to treat a patient. In recent years, tiny pieces of RNA called microRNAs have captured scientific attention for their potential as markers of health and disease.
A new study found that staff members who joined structured team debriefings after emergency care for children suffering in-hospital cardiac arrests improved their CPR performance and substantially increased the rates of patients surviving with favorable neurological outcomes.
Teleflex Inc.’s subsidiary Hotspur Technologies Inc. received U.S. Food and Drug Administration (FDA) 510(k) clearance to market the Arrow GPSCath Balloon Dilatation Catheters designed for use with 0.014-inch guidewires and in 150-cm length.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
August 11, 2014 — Medtronic announced it has received U.S. Food and Drug Administration (FDA) approval for the Attain Performa model 4298 quadripolar lead, and the Viva Quad XT and Viva Quad S cardiac resynchronization therapy defibrillators (CRT-D). The quadripolar lead and devices will be broadly available to physicians in mid-September.
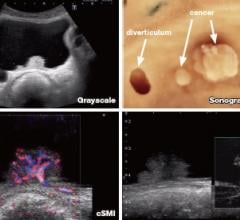
August 11, 2014 — Toshiba America Medical Systems is making it possible for clinicians to use ultrasound to see the smallest vessels in and around areas like tumors and lymph nodes, giving them a new way to diagnose disease faster and noninvasively.
August 11, 2014 — NeoCoil LLC announced it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market its new stand-alone wireless headphones and patient alert system. The NeoCoil wireless two-way audio system is NeoCoil's first wireless patient communication and entertainment system designed specifically to work in the magnetic resonance imaging (MRI) environment.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Imaging experts from the American Society of Echocardiography (ASE) released a paper, "Guidelines for the Cardiac Sonographer in the Performance of Contrast Echocardiography: A Focused Update from the American Society of Echocardiography,” to help clinicians optimize and demystify the use of ultrasound contrast media.
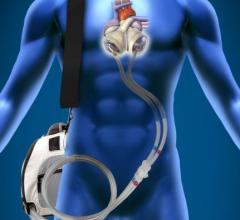
SynCardia Systems Inc. received approval in July from the U.S. Federal Drug Administration (FDA) for the SynCardia temporary Total Artificial Heart with SynHall valves, giving the company control over the last key component to manufacture the Total Artificial Heart
Running for only a few minutes a day or at slow speeds may significantly reduce a person’s risk of death from cardiovascular disease compared to someone who does not run, according to a clinical study published in the Journal of the American College of Cardiology.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
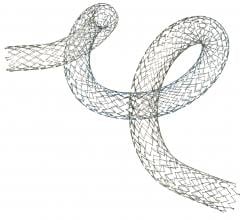
Biotronik announced the completion of patient enrollment in the superficial femoral artery (SFA) arm of its BIOFLEX-I clinical trial, an U.S. Food and Drug Administration (FDA)-approved investigative device exemption (IDE) trial evaluating the use of self-expanding nitinol stents in treating peripheral artery disease.
August 7, 2014 — The Berlin Heart Group announced they have completed enrollment in their post-approval study, the only condition of the humanitarian device exemption (HDE) approval that Berlin Heart received for the Excor pediatric ventricular assist device (VAD) on Dec. 16, 2011.

August 7, 2014 — A newly released study by IMV Medical Information Division shows cardiology departments in U.S. hospitals are moving beyond separate cardiovascular image management systems (cardiac picture archiving and communications systems [PACS]) and information systems (CVIS), with an eye to the improved efficiency provided by integration with other capabilities such as radiology PACS, other departmental IT systems and the hospital’s EMR (electronic medical records) system.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Transluminal Technologies LLC, a Syracuse, NY-based medical device company, received CE mark approval for the velox CD Vascular Closure Device.
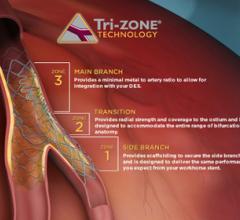
Tryton Medical Inc. announced that the first patient has been enrolled in the EXTENDED Access Registry (Tryton IDE XA registry), a single arm clinical study of its Tryton Side Branch Stent.
August 6, 2014 — St. Jude Medical announced the Center for Medicare and Medicaid Services (CMS) has approved a new technology add-on payment (NTAP) for the CardioMEMS heart failure (HF) monitoring system.


 August 12, 2014
August 12, 2014