A quarter century ago, doctors treating patients with implanted cardiac pacemakers had a big problem. Their patients were outliving the complex electrical devices that gave them an acceptable quality of life.
May 8, 2014 — Abbott announced its catheter-based MitraClip therapy has received Health Canada approval, providing physicians in Canada with the treatment option that can significantly improve symptoms, disease progression and quality of life for certain people with mitral regurgitation (MR).

May 8, 2014 — The past year has been a turbulent one for U.S. physicians, according to the 2014 Practice Profitability Index (PPI). Approximately 5,064 physicians contributed their insights to the second annual PPI in March 2014.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Argon Medical Devices Inc. began marketing the CelanerXT rotational thrombectomy system as a new addition to the Cleaner family of dialysis products.
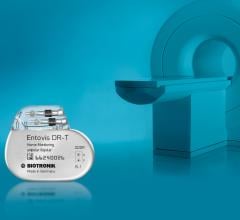
The Food and Drug Administration (FDA) has granted Biotronik approval for its Entovis pacemaker system with ProMRI technology.
May 7, 2014 — Cardiotek B.V. today announced it will commence commercialization of its EP-Tracer system following the 2014 Heart Rhythm Society (HRS) annual scientific sessions in San Francisco.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
May 7, 2014 — Women face greater limits on their lifestyle and have more severe symptoms as a result of peripheral artery disease (PAD), but minimally invasive procedures used to unclog arteries are just as successful as in men. The success of procedures, such as angioplasty or stent placement, in treating women with leg PAD was revealed in a Journal of the American College of Cardiology study.
HeartWare Intl. Inc. issued a voluntary Urgent Medical Device Correction related to all HeartWare Ventricular Assist System batteries, product codes 1650 and 1650-DE.
GE Healthcare received U.S. Food and Drug Administration (FDA) clearance for its Discovery IGS 740, a new rail-free mobile angiography system with a 41x41 cm detector.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...

The need for improved interoperability between healthcare IT systems and medical devices has been a growing problem in an increasingly integrated medical field. This is especially true in light of U.S. healthcare reform requirements, meaningful use demands and new rules requiring software integrations to receive reimbursements from the Centers for Medicare and Medicaid Services (CMS). Physicians are trained to treat patients, not to deal with IT integration issues, and many ask why they cannot access information they need from the Internet from anywhere, the same way they do on smartphones or their home computers. Until recently, this same level of connectivity has been elusive with their own hospitals’ IT systems. Over the past couple years, vendors have answered this question by launching Web-based
The past year saw the release of several new, innovative technologies to improve nuclear imaging in both positron emission tomography (PET) and single-photon emission computed tomography (SPECT).
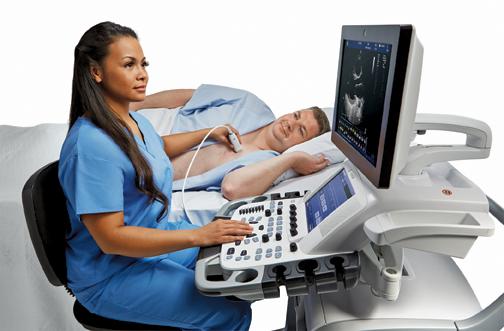
Cardiac ultrasound technology has advanced to keep up with several trends. These include improved workflow for greater efficiency, expanded use of qualification metrics, expanded use of 3-D echo to speed exam times and improve operator reproducibility, and expanded use of 3-D transesophageal echo (TEE) to aid guidance in the growing area of transcatheter structural heart procedures. Here are a few examples of how the newest technology is addressing these trends.
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
May 6, 2014 — Medtronic announced the first U.S. implant of the Evera MRI SureScan implantable cardioverter-defibrillator (ICD) system, following U.S. Food and Drug Administration (FDA) approval for its investigational device exemption (IDE) application and pivotal clinical trial protocol.

May 6, 2014 — Google Glass and its potential to improve patient care will be the focus of a first-of-its-kind special session at the 2014 Society for Cardiovascular Angiography and Interventions (SCAI) scientific sessions in Las Vegas.

The American College of Cardiology (ACC) annual meeting is a key event where vendors launch new cardiology technologies. After walking the entire expo floor at ACC.14, these are my choices for some of the most innovative new technologies that were on display.


 May 09, 2014
May 09, 2014