December 29, 2015 — Although using the radial artery as the access point for angioplasty has been linked to reduced ...
December 28, 2015 — National Cheng Kung University (NCKU) in Taiwan and Duke University School of Medicine are ...
December 28, 2015 — Toshiba announced Dec. 21 it is considering selling off its healthcare division as part of its ...
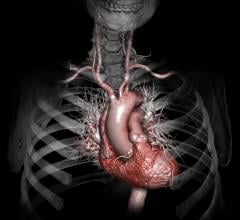
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
December 28, 2015 — The U.S. Food and Drug Administration (FDA) said Boston Scientific has started a Class I recall of ...
Leading cardiologist Valentin Fuster, M.D., Ph.D., has developed a free mobile application called “Circle of Health” to empower individuals around the globe to take action to comprehensively assess and enhance their daily overall heart health.
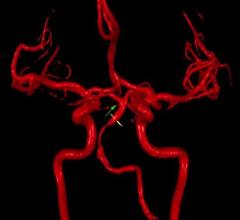
Patients who have had a stroke in the back of the brain are at greater risk of having another within two years if blood flow to the region is diminished, according to results of a multicenter study led by researchers at the University of Illinois at Chicago (UIC).
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...

Statins are lifesaving medications — they have been shown to greatly reduce heart attack and other related events by ...
Trinity Biotech plc announced submission of its Meritas Point of Care Analyzer and Meritas cardiac troponin-I (cTnI) point-of-care assay for U.S. Food and Drug Administration (FDA) 510(k) clearance.
December 22, 2015 - The U.S. Food and Drug Administration has cleared the Zoll LifeVest Wearable Cardioverter ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
December 22, 2015 — The American College of Cardiology (ACC) has launched a new Society of Thoracic Surgeons (STS)/ACC ...
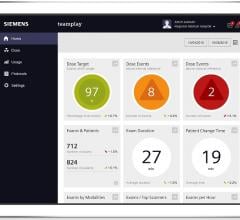
At the 101st Scientific Assembly and Annual Meeting of the Radiological Society of North America (RSNA), Siemens displayed new offerings for teamplay, a cloud-based network that helps link hospitals and healthcare experts, enabling them to exchange data and pool their knowledge.
December 22, 2015 — In 2015, results from the ITALIC and CvLPRIT trials, along with studies examining lifestyle factors ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
December 22, 2015 — Biotronik received U.S. Food and Drug Administration (FDA) approval for use of its latest family of ...
Siemens Healthcare’s approach to delivering dose-neutral dual energy images obtained on Somatom computed tomography (CT) scanners using TwinBeam Dual Energy technology is now commercially available on the Somatom Definition Edge and Somatom Definition AS CT systems.
Carestream Health showcased its MyVue Center Self-Service Kiosk that enhances patient access and sharing of their medical exam records at the 2015 Radiological Society of North America (RSNA) conference.

 December 29, 2015
December 29, 2015