June 3, 2025 — Bayer announced it will present multiple new analyses of the Kerendia (finerenone) clinical trial program ...
May 30, 2025 — BiVACOR, a clinical-stage medical device company developing the world’s first titanium Total Artificial ...
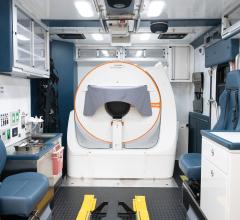
May 28, 2025 — Siemens Healthineers has introduced the first mobile stroke unit (MSU) featuring the Somatom On.site head ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
May 27, 2025 — Abbott has announced the U.S. Food and Drug Administration (FDA) has approved the company's Tendyne ...
May 27, 2025 — CeleCor Therapeutics has completed its multinational Phase 3 clinical trial of DisaggproT (zalunfiban) ...
May 27, 2025 — Despite scientific advances in cardiovascular care, people in living in rural areas and other communities ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
May 22, 2025 — Auxira Health has officially launched — the company provides a new approach to clinical support purpose ...
May 21, 2025 — Corify Care recently announced it has entered into a know-how agreement with Mayo Clinic. Corify Care is ...
May 21, 2025 – Johnson & Johnson MedTech has announced the U.S. launch of the Soundstar Crystal Ultrasound Catheter for ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
May 20, 2025 — Shockwave Medical, Inc., part of Johnson & Johnson MedTechhas announced the 30-day primary endpoint ...
May 15, 2025 — Abundant Venture Partners has launched the Abundant Platform to grow high value, sustainable companies ...
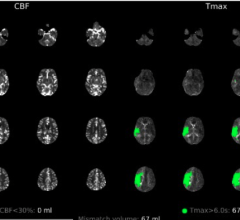
May 21, 2025 — RapidAI recently announced new study findings that show RapidAI’s clinically deep algorithms deliver ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
May 20, 2025 — Royal Philips has launched the RADIQAL (Radiation Dose and Image Quality Trial) trial. This multicenter ...
May 19, 2025 — Gradient Denervation Technologies recently announced the company’s pulmonary denervation system has ...
May 13, 2025-- GE HealthCare recently announced the U.S. Food and Drug Administration (FDA) has approved a pediatric ...


 June 04, 2025
June 04, 2025