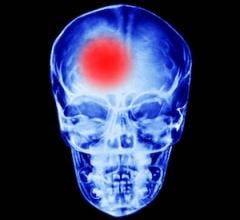
Jan. 15, 2026 — Boston Scientific Corp. and Penumbra, Inc. have entered into a definitive agreement under which Boston ...
Jan. 16, 2026 — MemorialCare Heart & Vascular Institute has launched the FDA-approved Symplicity Renal Denervation (RDN) ...
Jan. 6, 2026 — UltraSight, a provider of AI-guided cardiac imaging workflows, has announced FDA clearance to expand its ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Jan. 13, 2026 – Innovative Health, Inc. has received its 50th clearance from FDA to reprocess single-use medical devices ...
Jan. 13, 2026 — A streamlined, nurse-led response for hospitalized patients experiencing an acute stroke at a Texas ...
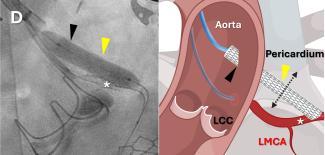
In a world first, a team of researchers at the National Institutes of Health (NIH) and Emory School of Medicine, Atlanta ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Jan. 8, 2026 — AccurKardia recently announced U.S. Food and Drug Administration (FDA) 510(k) clearance and the ...
Jan. 13, 2026 — AliveCor has received U.S. Food and Drug Administration (FDA) clearance for the next generation of KAI ...
Jan. 13, 2026 — Kestra Medical Technologies, a wearable medical device and digital healthcare company, has announced a ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Medtronic has announced the commercial launch of the OmniaSecure defibrillation lead in the U.S., with first cases being ...
Jan. 8, 2026 — Compremium AG recently announced that the U.S. Food and Drug Administration (FDA) has granted ...
Jan. 12, 2026 — YorLabs, Inc., a medical technology company providing next-generation intracardiac imaging solutions for ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Jan. 12, 202 — Abbott and AtaCor Medical have announced a collaboration to advance a next-generation investigational ...
Jan. 12, 2026 — HeartLung Corp. has announced U.S. Food and Drug Administration (FDA) clearance of AI-CVD, its AI ...
Jan. 6, 2026 — Millions of Americans living with heart failure are not receiving medications that have been proven for ...


 January 19, 2026
January 19, 2026