All of the major vendors in the United States introduced new systems and technologies in the past few years to reduce dose and enhance visualization in the cath lab. The vendors have tailored their systems into various models for specific specialties and at various price points depending on the degree of functionality. Most vendors also offer software to enhance visualization of stents and other devices.
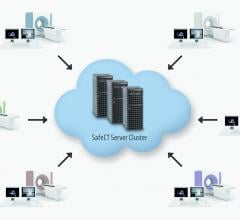
Medic Vision Imaging Solutions Ltd. announced the installation of SafeCT (computed tomography) at St. Joseph’s/Candler Health System, Savannah, Georgia. The healthcare system’s two hospitals and three outpatient facilities are all served by a centralized SafeCT Enterprise system.
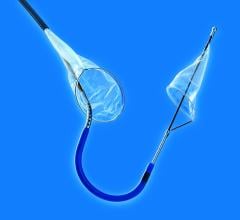
Conavi Medical Inc. (formerly Colibri Technologies Inc.) has received U.S. Food and Drug Administration (FDA) 510(k) clearance for the Foresight ICE System. The system is cleared for intracardiac and intraluminal ultrasound visualization of cardiac and great vessel anatomy, as well as visualization of other devices in the heart and great vessels of patients.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Heartbeats can now be measured without placing sensors on the body, thanks to a new technology developed in Japan. Researchers at the Kyoto University Center of Innovation, together with Panasonic Corp., have come up with a way to measure heartbeats remotely, in real time, and under controlled conditions with as much accuracy as electrocardiographs.
January 26, 2016 — In a breakthrough that could change the future of pacemakers, Technion-Israel Institute of Technology ...
January 26, 2016 — Fujifilm SonoSite Inc. announced CE mark and U.S. Food and Drug Administration (FDA) 510(k) clearance ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Now, medical technology purchasers and users have an instant online forum to rate and review technologies they are using, with Rate This Model, the newest enhancement to the ECRI Institute’s SELECTplus capital decision support solutions suite.
St. Jude Medical is recalling the Optisure implantable cardioverter defibrillator (ICD) leads due to a manufacturing error that may have caused damage to the insulation layer of one of the shock coils. Depending on device programming and the depth of the cut, this could result in the inability of the defibrillator to deliver electrical therapy to the patient. The use of affected products may cause serious adverse health consequences, including patient injury or death.
The suspension of the medical device excise tax will have positive consequences for the U.S. medical device market over the next few years, says an analyst with research and consulting firm GlobalData.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
New recommendations from the American College of Cardiology (ACC) and the American College of Radiology (ACR) have established appropriate use of diagnostic imaging for patients with chest pain, one of the most common reasons for emergency department visits.
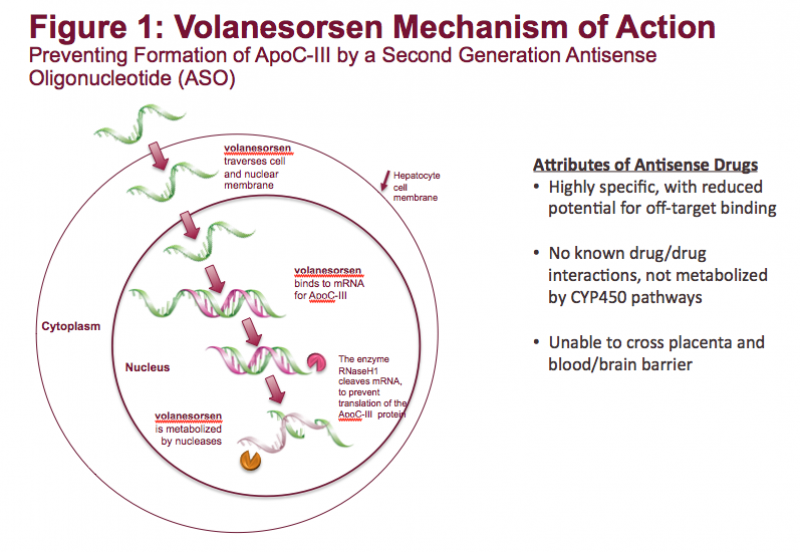
Familial chylomicronemia syndrome (FCS) is a rare, genetic disease characterized by mutations in genes affecting the ...
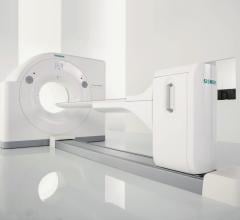
Siemens Healthcare has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for the Biograph Horizon positron emission tomography/computed tomography (PET/CT) system, which offers premium performance at a low total cost of ownership.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
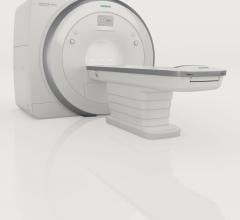
Siemens Healthcare announced that the U.S. Food and Drug Administration (FDA) has cleared the Magnetom Amira 1.5 Tesla magnetic resonance imaging (MRI) system.
This case study is from the Cardiac Imaging Department, Hospital Clinic, Barcelona, Spain. A woman in her early 60s came ...
GE Healthcare is taking the next leap in image quality performance, quantification and workflow with the introduction of ...

 January 27, 2016
January 27, 2016