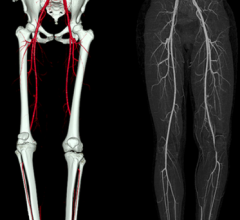
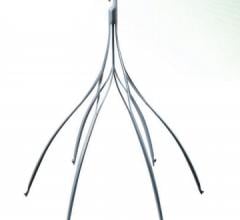
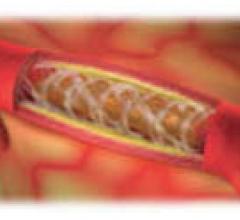
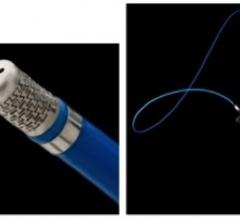
Six-month results of the ESPRIT trial suggest a bioresorbable drug-eluting scaffold is effective in opening blocked blood vessels in the legs and pelvis, as presented at the 26th annual International Symposium on Endovascular Therapy (ISET).
Peripheral artery disease (PAD) sufferers maintain improved quality of life, including being able to walk farther, three years after being treated with stents to open their blocked leg arteries, according to STROLL trial results presented at the 26th annual International Symposium on Endovascular Therapy (ISET).

The U.S. Food and Drug Administration (FDA) has approved many cardiac implantable electronic device models currently in use through a review process in which models were deemed safe and effective based on approval of prior versions of the device, according to a study published in JAMA.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Argon Medical Devices Inc. received clearance from the U.S. Food and Drug Administration (FDA) to begin marketing the OptionElite retrievable inferior vena cava (IVC) filter with an over-the-wire delivery technique.
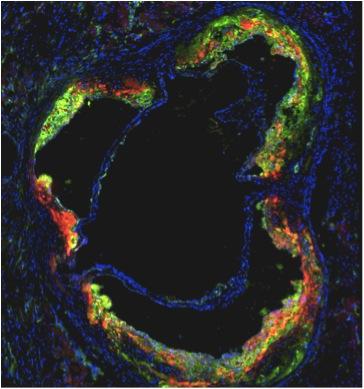
An international research team, led by Icahn School of Medicine at Mount Sinai investigators, designed and tested a high-density lipoprotein (HDL) nanoparticle loaded with a statin drug. In mouse studies, they show HDL nanotherapy is capable of directly targeting and lowering dangerous inflammation in blood vessels.
An international research team at Icahn School of Medicine at Mount Sinai is testing a sugar-based tracer contrast agent to be used with positron emission tomography (PET) imaging to help identify dangerous inflammation and high-risk vulnerable atherosclerotic plaque inside vessel walls that causes acute heart attacks and strokes.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
An implant of the ReZolve2 bioresorbable scaffold was transmitted live via satellite at the Transcatheter Cardiovascular Therapeutics (TCT) 2013 conference in San Francisco.
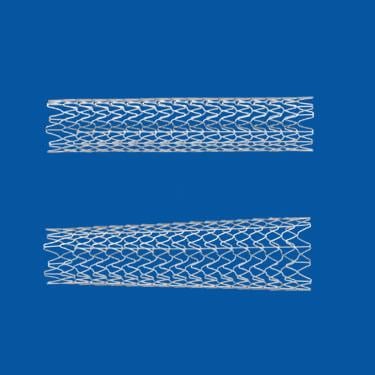
The three most common systems to place stents in blocked carotid arteries of the neck have similarly low rates of complication and death among U.S. patients, according to a study published by JACC: Cardiovascular Interventions.
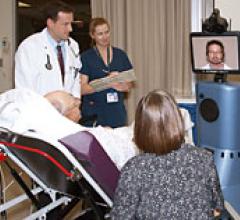
Research published in the American Journal of Managed Care indicated using telemedicine to deliver stroke care, or telestroke, appears to be cost-effective for society. Telestroke robots allow stroke patients in facilities with no neurology specialists to receive real-time consultation from neurologists in other areas via computer.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
The U.S. Food and Drug Administration (FDA) gave market clearance to Irvine Biomedical Inc., a St. Jude Medical company, for its Therapy Cool Flex Ablation Catheter and IBI 1500T9-CP v.1.7 Cardiac Ablation Generator.
The U.S. Food and Drug Administration (FDA)’s Cardiovascular and Renal Drugs Advisory Committee recommended approval of vorapaxar, Merck’s investigational antiplatelet medicine for the reduction of atherothrombotic events, when added to standard of care in patients with a history of heart attack and no history of stroke or transient ischemic attack.
The U.S. Food and Drug Administration's (FDA) Cardiovascular and Renal Drugs Advisory Committee voted against the approval of the use of oral anticoagulant Xarelto (rivaroxaban) to reduce the risk of thrombotic cardiovascular events in patients with Acute Coronary Syndrome (ACS) in combination with standard antiplatelet therapy. Janssen Research & Development LLC sought approval of rivaroxaban at a proposed dose of 2.5 mg twice daily (BID) for a 90-day treatment duration.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
The first commercial implant of Elixir Medical’s CE Mark-approved DESolve Novolimus Eluting Coronary Scaffold was performed in Germany. Elixir’s fully bioresorbable DESolve scaffold for coronary artery disease restores blood flow to the heart like metallic stents and then dissolves, resulting in a functional artery free of a permanent implant.
BioVentrix’s Revivent-TC Ventricular Enhancement System was implanted in a man via Less Invasive Ventricular Enhancement (LIVE) in Prague, Czech Republic. The procedure, which is used to reshape and reduce the left ventricle, was performed on a 64-year-old man suffering from ischemic heart failure.
BeneChill Inc. received the 2014 Frost & Sullivan Award for Technology Innovation Leadership for its RhinoChill intranasal cooling system. BeneChill's RhinoChill is a nasopharyngeal device that can induce and sustain therapeutic hypothermia during active resuscitation after cardiac arrest. The system is noninvasive, time critical and can be used by nonmedical personnel to prevent brain damage associated with cardiac arrest.


 January 23, 2014
January 23, 2014