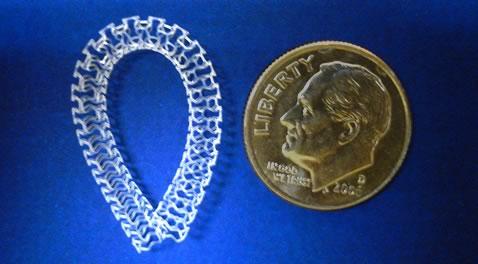
Amaranth Medical announced the closing of an equity investment by Boston Scientific Corp.

Edwards Lifesciences Corp. announced that it received investigational device exemption (IDE) approval from the U.S. Food and Drug Administration (FDA) to initiate a clinical trial of the Sapien 3 transcatheter aortic heart valve in the treatment of intermediate risk patients with severe symptomatic aortic stenosis.
MediValve Ltd. announced that it has received clearance of a Pre-Marketing Notification Application (510(k)) from the U.S. Food and Drug Administration (FDA) for its acWire Guidewire.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
A clinical trial found that Xa inhibitor edoxaban met the primary efficacy endpoint of non-inferiority compared to warfarin for the prevention of stroke or systemic embolic events (SEE) in patients with non-valvular atrial fibrillation (NVAF).
CVRx Inc. announced findings from a health-economic analysis published in the Journal of Hypertension that indicates Barostim Therapy is a cost-effective treatment option for patients with drug-resistant hypertension.
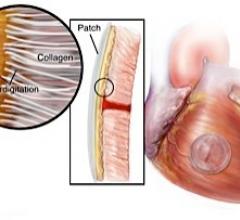
In a preclinical study, researchers from Boston Children's Hospital, BWH and Massachusetts Institute of Technology (MIT) developed a bio-inspired adhesive that could rapidly attach biodegradable patches inside a beating heart in the exact place where congenital holes in the heart occur.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
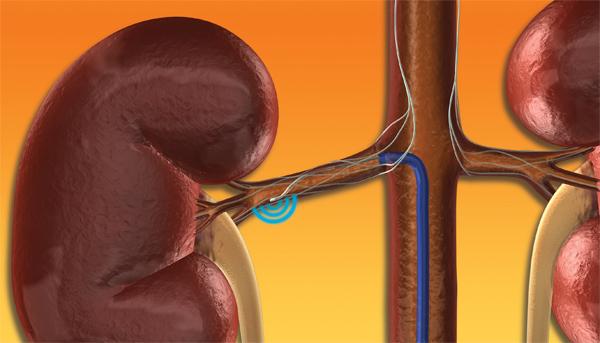
Medtronic Inc. announced its U.S. pivotal trial for its Symplicity renal denervation system to treat resistant hypertension, the SYMPLICITY HTN-3 trial, failed to meet its primary efficacy endpoint. The trial met its primary safety endpoint, and the trial's Data Safety Monitoring Board (DSMB) concluded that there were no safety concerns in the study.
SunTech Medical, a Halma company that provides noninvasive blood pressure monitoring products and technologies, has signed a cooperation contract with Corscience GmbH and Co. KG, medical engineering company that specializes in cardiovascular applications, Erlangen, Germany.

Topera Inc. received U.S. Food and Drug Administration (FDA) 510(k) clearance for the latest generation of its 3D Mapping System.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
iRhythm Technologies announced that a prospective study by Scripps Translational Science Institute (STSI) has found that use of the company's ZIO Service significantly increased detection of cardiac arrhythmias, compared to use of the traditional Holter monitor.
Biodex Medical Systems Inc. introduced its line of ultrasound tables, which feature redesigned optional side rails that tuck underneath the table when not in use to allow clear access to the tabletop.
A report by MarketsandMarkets analyzes and studies the major technology and market drivers, restraints and opportunities in North America, Europe, Asia and Rest of the World.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Vasomedical Inc. announced that the FDA issued its final order Dec. 30, 2013, reclassifying external counterpulsation (ECP) devices for treatment of chronic stable angina for patients that are refractory to antianginal medical therapy and without options for revascularization from class III to class II (special controls).
The winners of the Right Dose Image Contest were announced at the Radiological Society of North America Annual Meeting (RSNA 2013) in Chicago.
Stentys, developer of the world's first self-apposing stent to treat acute myocardial infarction (AMI), announced new data in indications outside of myocardial infarction.

 January 14, 2014
January 14, 2014