Neovasc Inc. reported the results from its COSIRA (coronary sinus reducer for treatment of refractory angina) trial assessing the efficacy and safety of the Neovasc Reducer, a percutaneous device for the treatment of refractory angina.
Esaote will showcase Prevention Suite, a unique package of ultrasound imaging modalities for both cardiac and vascular investigations at the EuroEcho-Imaging 2013 conference Dec. 11-14 in Istanbul, Turkey.

The use of renal stenting in combination with medication-based therapy to treat hypertension showed no improvement in patient outcomes over the use of medications alone in treating hypertension. The results from the highly-anticipated CORAL trial were presented at the American Heart Assn. (AHA) 2013 Scientific Sessions in Dallas.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...

AtriCure Inc., a developer of solutions to treat atrial fibrillation, announced the U.S. Food and Drug Administration’s (FDA’s) approval to begin enrollment in a clinical study of AtriCure’s AtriClip Left Atrial Appendage Exclusion System.
Five global MRI manufacturers—in the United States, Europe and Asia—have selected ContextVision’s GOPView MRI2Plus to upgrade their MRI equipment. Today’s healthcare industry requires improved efficiencies without sacrificing patient safety. As radiologists around the world review an increasing number of MRI images every day, image quality is key.

Fractional flow reserve (FFR) is considered the gold standard in determining the hemodynamic significance of coronary lesions, but its use remains low. Two new technologies may help increase its use.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...

A German court ordered Nov. 14 the discontinuation of a prior court ruling that prohibited Medtronic from commercially marketing or selling the CoreValve transcatheter aortic valve replacement (TAVR) system in Germany since Aug. 26, 2013.
GE Healthcare will introduce new software to help humanize magnetic resonance imaging (MRI) with the DV24.0 Continuum Pak Silent Scan noiseless MRI at the Radiological Society of North America 2013 Annual Meeting (RSNA 2013) in Chicago.
The first two commercial implants of the Boston Scientific Lotus Valve System have taken place in a German hospital.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
The U.S. Food and Drug Administration (FDA) has classified the Medtronic’s recently initiated voluntary recall of certain interventional guidewires as a Class I recall.
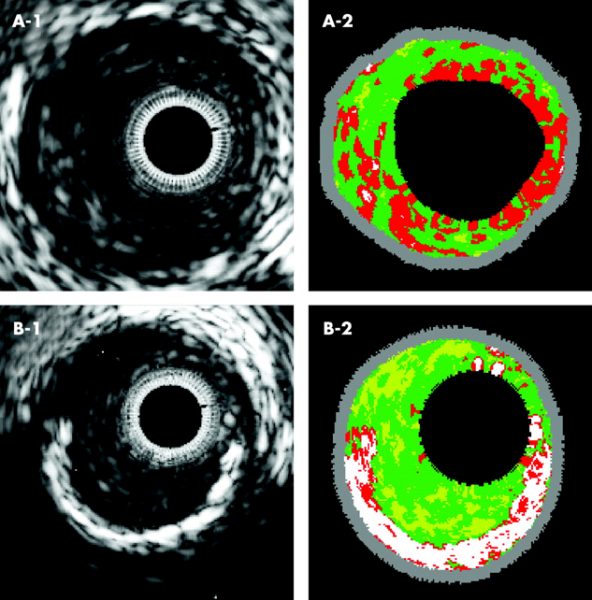
In an expert consensus document e-published in Catheterization and Cardiovascular Interventions (CCI), the Society for Cardiovascular Angiography and Interventions (SCAI) offers recommendations for optimal use of three technologies that interventional cardiologists often use to assess the severity of blockages in heart arteries.
The American College of Cardiology (ACC) and the American Heart Association (AHA) released a clinical practice guideline to help primary care clinicians better identify adults who may be at high risk for developing atherosclerotic cardiovascular disease, stroke and who thus may benefit from lifestyle changes or drug therapy to help prevent it.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Resverlogix Corp. announced two additional results from the ongoing analysis of its Phase 2b ASSURE clinical trial using intravascular ultrasound (IVUS) to study high-risk cardiovascular disease (CVD) patients for assessing benefits of RVX-208.
A study conducted in Ireland found that aspirin does not adequately protect 20 percent of patients with established heart disease.
Many healthcare systems are finding their general PACS and cardiology PACS are both aging and their image storage is reaching capacity. How do the diagnostic imaging departments address the needs of their cardiologists and radiologists?


 November 20, 2013
November 20, 2013