Based on its recent analysis of the cardiology informatics market, Frost & Sullivan recognizes Ascend HiT with the 2013 North America Frost & Sullivan Award for Customer Value Leadership. With the introduction of CardioAnalytics, Ascend has proved that it is at the vanguard of the cardiology market's efforts to enhance quality and analytics. This product complements and extends the best-of-breed Ascend CV reporting products, and in the process, positions the company to deliver one of the most crucial elements of the cardiology value chain—cardiovascular procedural reporting.
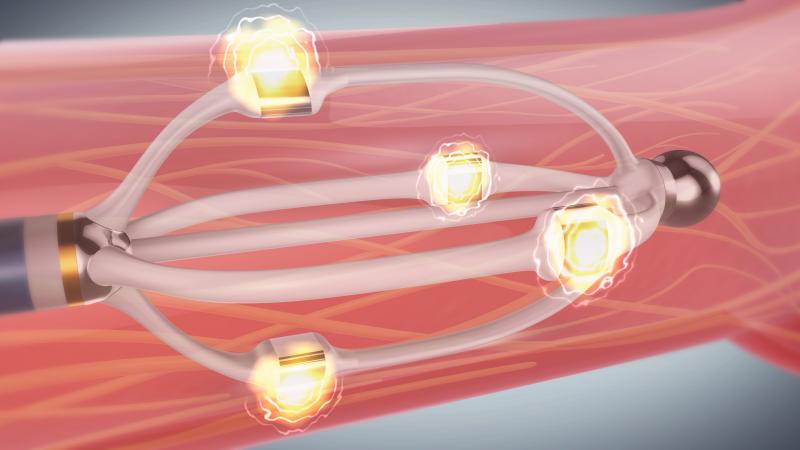
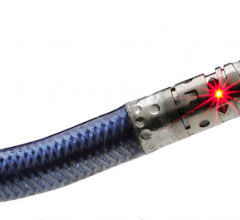
September 3, 2013 — St. Jude Medical Inc. announced the CE mark approval of its next-generation EnligHTN renal denervation system for treating patients with drug-resistant, uncontrolled hypertension. The EnligHTN system was display during the 2013 European Society of Cardiology (ESC) meeting Aug. 31 to Sept. 3.
St. Jude Medical Inc. today announced U.S. Food and Drug Administration (FDA) approval and first use of MediGuide Enable Ablation Catheters. The ablation catheters, which are used to treat specific irregular heartbeats, expand the MediGuide platform for St. Jude Medical.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Avinger Inc. has received CE Mark approval for Pantheris – a system that combines directional atherectomy capabilities with real-time intravascular visualization to remove plaque from blocked arteries. Pantheris is designed to remove the blockage while avoiding the disruption of normal arterial wall structures. This new type of image-guided atherectomy is referred to as lumectomy. Currently, approximately 200 million patients worldwide suffer from peripheral artery disease (PAD).
A listing of remote image viewing systems being shown at RSNA 2013
Ascendian Healthcare Consulting has announced a comprehensive vendor neutral archive (VNA) acceleration solution that enables healthcare organizations rapid accessibility, efficiency and shared utilization of medical imaging assets.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
August 30, 2013 — PFM Medical received U.S. Food and Drug Administration (FDA) premarket approval (PMA) for its Nit-Occlud Patent Ductus Arteriosus (PDA) device, a permanently implanted prosthesis indicated for percutaneous, transcatheter closure of small to moderate size patent ductus arteriosus.
Philips Healthcare launched the Epiq ultrasound system, a first-of-its-kind ultrasound architecture that offers a new approach to creating ultrasound images. Making its debut at the European Society of Cardiology (ESC) 2013 Congress in Amsterdam, Epiq features a new imaging technology called nSIGHT that, when combined with Philips' new Anatomical Intelligence technology, delivers faster speed and improved image clarity. It has received 510(k) clearance from the U.S. Food and Drug Administration (FDA).
To counter the significant levels of morbidity and mortality associated with pericardial disease (disease of the sac around the heart), experts from the American Society of Echocardiography (ASE), the Society for Cardiovascular Magnetic Resonance (SCMR), and the Society of Cardiovascular Computed Tomography (SCCT) came together to review evidence and provide future guidance to clinicians. For the first time, an expert consensus statement on the appropriate use of multimodality imaging in the diagnosis and management of pericardial diseases will be published in the September issue of the Journal of the American Society of Echocardiography (JASE). The writing group was chaired by Allan L. Klein, M.D., FASE, director of Cardiovascular Imaging Research and the Pericardial Center and an echocardiographer from the esteemed Cleveland Clinic in Cleveland, Ohio.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Digirad Corp. and Dilon Diagnostics have signed an exclusive international distribution agreement for Dilon to distribute Digirad's lines of nuclear imaging cameras. .
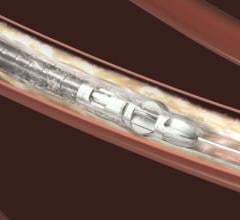
Bayer HealthCare announced that the U.S. Food and Drug Administration (FDA) has cleared two new Jetstream atherectomy catheters. The SC (Single Cutter) atherectomy catheter is available in 1.6-mm and 1.85-mm cutting diameters. The XC (eXpandable cutter) atherectomy catheter features both front cutting tip and expandable blade technology for use in larger vessels. The XC is available in two cutting diameters: 2.1-mm blades down / 3.0-mm blades up and 2.4-mm blades down / 3.4-mm blades up.

August 28, 2013 — Effective Oct. 1, Cook Medical’s Zilver PTX drug-eluting peripheral stent qualifies for new-technology add-on payments under Medicare’s hospital inpatient prospective payment system.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
According to Millennium Research Group (MRG), efforts to streamline the regulatory approval process in Japan have resulted in the recent entry of numerous devices in the Japanese peripheral vascular (PV) device market, and more products, such as drug-coated balloons and infrapopliteal stents, are expected to enter in the next few years.
Experts have found that radiation dosage can have serious complications for patients with cancer. Cancer patients who receive chest radiation should be evaluated for heart disease before beginning radiation, and every five to 10 years afterward, according to the American Society of Echocardiography (ASE) and the European Assn. of Cardiovascular Imaging (EACVI) of the European Society of Cardiology (ESC). This expert consensus statement on the use of imaging to detect radiation-induced heart disease (RIHD) will be published in the September issue of the Journal of the American Society of Echocardiography (JASE). The writing group was co-chaired by Vuyisile Nkomo, M.D., an echocardiographer from the renowned Mayo Clinic in Rochester, Minn.
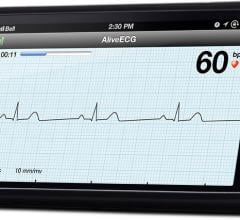
AliveCor Inc. released a new AliveECG App for its Heart Monitor that features an Enhanced Filter, a noise cancelling technology that improves the visual output of the ECG. The AliveCor Heart Monitor is a mobile device-based ECG monitor for the iPhone 4, 4S and 5 that is used to record, display, store and transfer single-channel electrocardiogram (ECG) rhythms.

 September 04, 2013
September 04, 2013