Safire Duo and Cool Path Duo ablation catheters
September 3, 2013 – St. Jude Medical Inc. announced U.S. Food and Drug Administration (FDA) approval and first use of MediGuide Enable Ablation Catheters. The ablation catheters, which are used to treat specific irregular heartbeats, expand the MediGuide platform for St. Jude Medical.
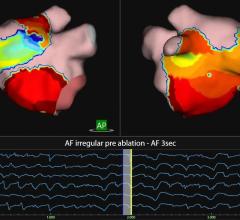
The MediGuide Enabled ablation catheters can be visualized using 3-D magnetic tracking. MediGuide technology is the first and only system to potentially reduce the duration of radiation exposure during catheter ablation procedures. The catheters are used to create lesions during cardiac ablation procedures to treat atrial flutter, a heart rhythm disorder where the upper chambers of the heart beat too fast and out of sync with the lower chambers. MediGuide sensors allow the catheters to be visualized and navigated in real-time on pre-recorded fluoroscopy.
The Safire Duo and Cool Path Duo irrigated tip ablation catheters can now be visualized and navigated in real-time on pre-recorded fluoroscopy with MediGuide Technology. Featuring a high-performance tip with uni-directional or bi-directional deflection, these ablation catheters have the ability to be steered in two different directions for improved reach and maneuverability. They also include 12 irrigation ports for cooling tissue during procedures.
“The new MediGuide Enabled Ablation Catheters expand the utility of the MediGuide System, which is an important platform that provides clinicians with the ability to reduce the duration of radiation exposure and improve the accuracy and consistency of procedures. The MediGuide Technology is an example of our commitment to providing solutions for expensive and epidemic diseases that can have a direct impact on both physicians and patients,” said Frank J. Callaghan, president of the St. Jude Medical cardiovascular and ablation technologies division.
The St. Jude Medical MediGuide Technology provides a pioneering solution to better manage fluoroscopy exposure during electrophysiology procedures. MediGuide Technology provides a comprehensive platform and set of tools to address a broad array of clinical applications including catheter ablations and CRT implants.
Worldwide, physicians perform several billion radiation-based imaging studies annually, approximately one-third of which are in cardiovascular patients. According to the American Heart Association, the collective dose of ionizing radiation that patients annually received during medical tests increased among the general population an estimated 600 percent between 1980 and 2006. As a result, there has been a dramatic increase in human exposure to ionizing radiation.
For more information: www.sjm.com


 September 05, 2025
September 05, 2025