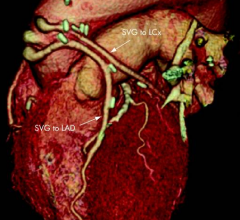
There have been several advances in electrophysiology (EP) technologies this past year, many of which were highlighted during the Heart Rhythm Society (HRS). These advances included a leadless pacemaker, new data on contact force sensing ablation catheters, a new electromapping system entering the market and new techniques to map and ablate atrial fibrillation (AF), said Jagmeet Singh, M.D., Ph.D., director, resynchronization and advanced cardiac therapeutics program at the new Mass General Institute for Heart, Vascular and Stroke Care. Singh also presented numerous sessions at HRS.
A clinical study of two sets of 894 matched emergency department (ED) patients presenting with chest pain revealed that the use of coronary computed tomographic angiography (CCTA) led to fewer hospital admissions and shorter ED stays. According to lead researcher Michael Poon, M.D., of Stony Brook University School of Medicine, the findings provide evidence that CCTA offers an alternative means of improving the triage of chest pain patients.
Vanderbilt Heart and Vascular Institute is participating in the VELOCITY study, a randomized controlled clinical study to assess the safety and feasibility of a rapid cooling system for heart attack patients that could minimize damage to the heart.
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Edwards Lifesciences received conditional investigational device exemption (IDE) approval from the U.S. Food and Drug Administration (FDA) to initiate a clinical trial of its Edwards Sapien 3 transcatheter aortic heart valve and accessories.
August 5, 2013 — AstraZeneca announced results from a new sub-analysis of the PLATO study that evaluated the incidence of stent thrombosis in patients with acute coronary syndrome (ACS).
Thubrikar Aortic Valve’s Optimum TAV, transcatheter aortic valve implantation (TAVI) system, has surpassed 200 million cycles in an ongoing durability test — which simulates over five years in humans and meets the requirement set by the International Organization for Standardization (ISO) — in a third party GLP study.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Abiomed Inc. reported that physicians have implanted more than 15,000 Impella pumps in U.S. patients requiring hemodynamic support. The 15,000th Impella procedure took place at Mercy General Hospital in Sacramento, Calif., with an Impella pump that provided prophylactic circulatory support.
The U.S. Food and Drug Administration (FDA) recently granted market clearance for a hand lotion designed to offer radiation protection from X-rays. Physicians such as interventional cardiologists who work with live angiographic fluoroscopic X-ray systems often have their hands in the radiation field during imaging. The cream, developed by radiation protection product company BloXR Corp., is applied prior to donning gloves, or over a glove with another glove on top, to serve as a lightweight radiation shield
Abbott announced results from a study evaluating its High Sensitive Troponin-I (hsTnI) assay. The study, conducted by researchers at Brigham and Women's Hospital, demonstrated that Abbott's hsTnI test (currently for research-use only in the United States) may help doctors predict which patients presenting with symptoms of a heart attack, such as severe chest pain, are at a higher risk for having a heart attack 30 days later.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
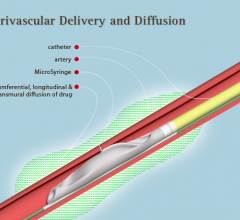
Mercator MedSystems has been issued U.S. Patent 8,465,752, which protects the company’s sole U.S. rights to deliver drugs or other nerve modifying agents to tissue surrounding arteries for renal denervation (RDN). The system also can be used for carotid body modulation (CBM) procedures.
InspireMD Inc. said the first patient has been enrolled in the Master II IDE clinical trial to evaluate the safety and effectiveness of the MGuard Prime Embolic Protection Stent (EPS) in patients suffering from ST elevation myocardial infarction (STEMI).
The Mikro-Cath disposable blood pressure catheter delivers accurate and reliable hemodynamic data from within the heart or vascular system for expert insight in advanced applications.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
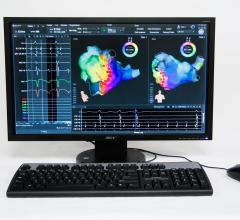
August 1, 2013 — Boston Scientific Corp. has received U.S. Food and Drug Administration (FDA) 510(k) clearance for the Rhythmia mapping system, a next-generation 3-D mapping and navigation solution for use in cardiac catheter ablations and other electrophysiology (EP) procedures to diagnose or treat a variety of conditions in which the heart beats abnormally.
Magnetic resonance imaging (MRI) scans of children who have had chemotherapy can detect early changes in their hearts, according to research findings in BioMed Central's open access journal Journal of Cardiovascular Magnetic Resonance.
Polymer Technology Systems Inc. has introduced the CardioChek Plus analyzer. It is a combination reflectance lipid and electrochemical glucose analyzer that delivers rapid test results for lipids and glucose simultaneously. The system has Wi-Fi capabilities and can transmit patient results to data collection applications.

 August 06, 2013
August 06, 2013