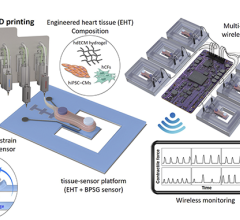
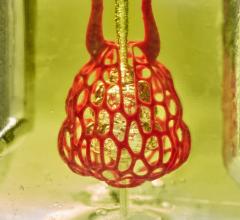
A 3-D printed vessel-like lumen made from living cells as part of the research at The South Carolina Project for Organ Biofabrication.
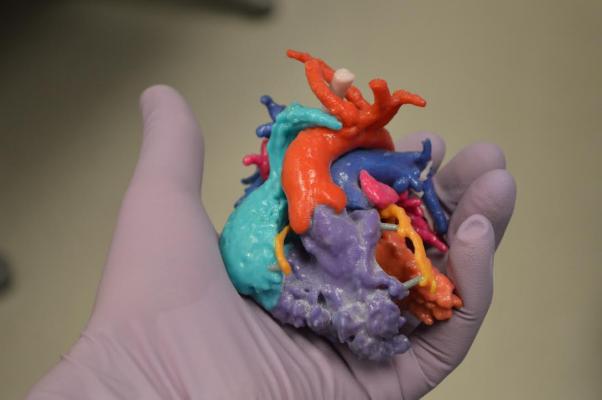
Science fiction offers a lot of ideas for creating new body parts on demand, and the advancement of 3-D printing (also called additive manufacturing) is slowly translating this idea into science fact. Today, the 3-D printed anatomic models created from patient computed tomography (CT), magnetic resonance imaging (MRI) or 3-D ultrasound imaging datasets are used for education and to plan and navigate difficult procedures. These models are used to teach about complex or rare cardiac or congenital conditions that up until recently could only be seen using examples extracted from cadavers. Today, anatomical models of rare cardiac anatomy can be printed on-demand from CT scans of surviving patients.
That concept can now be translated into 3D printing of implantable devices customized to a specific patient using their imaging. Experts at several medical conferences are saying printing functional biological replacement tissues is already in development. Three-dimensional printing has become a topic of discussion in conference sessions and on the expo floors at many medical meetings over the past several years. The topic was covered in a session at the Radiological Society of North America's (RSNA) annual meeting in December, which is detailed in the following sections.
Watch the VIDEO: Applications in Cardiology for 3-D Printing and Computer Aided Design, and interview with Dee Dee Wang, M.D., FACC, FASE, Henry Ford Hospital, explaining the use of 3D printing to aid procedural planning and guidance in complex structural heart cases.
Early Experience 3D Printing Implantable Devices
Printed 3-D models are currently used for surgical planning in complex cases, especially in pediatric congenital heart procedures, said Richard G. Ohye, M.D., professor of cardiac surgery, head, section of pediatric cardiovascular surgery, surgical director, pediatric cardiovascular transplant program, co-director, Michigan Congenital Heart Center, C.S. Mott Children's Hospital, Ann Arbor, Mich. However, he explained 3-D printing will soon allow the creation of customized implantable medical devices, including actual tissue or vessel replacements.
In fact, 3-D printed devices are already being used on a small scale. He presented a case of a three-month-old patient whose airway was underdeveloped and required a splint to hold it open. The patient underwent a CT scan and a 3-D reconstruction of the airway allowed doctors to create a virtual airway splint implant customized to fit into the small anatomy. The design included a “C”-shaped tube that had numerous holes to use as suture anchor points. The shape was designed to allow it to expand outward as the patient grew. They then 3-D printed the splint from bioresorbable plastic and implanted it in the patient. Ohye said the material it was made from is expected to dissolve within three to four years.
The Finnish dental equipment maker Planmeca recently introduced a 3-D printer that allows dental laboratories and large clinics to create dental splints, models and surgical guides. In the near future, the Planmeca Creo printer will also support the creation of intricate, customized temporary fillings.
The jump to printing full organs to transplant is much more complex, but the groundwork is being laid. Ohye said engineered heart tissue created using cardiac stem cells has already been created, but it is limited to a size of about 200 microns. Anything larger requires blood vessels to keep the cells alive, he explained.
3-D Printing of Biological Tissue Implants
Research is being conducted to enable 3-D printing of blood vessels, where cells are deposited by the robotically driven printer in patterns that build up layer-by-layer to create a lumen. That same concept is being tested at a few centers to create 3-D print heart valves. Ohye said the process currently being investigated uses a printed matrix of biocompatible material, in which stem cells can then be deposited. If the process can be worked out to create engineered, printed organs, these might be used to create benchtop model organs for new drug testing in the next few years. Implantable 3-D printed living organs for transplant into human patients are also a very real possibility.
“Bioprinting is likely to be a huge field for the future of medicine,” said Roger Markwald, Ph.D., director, Cardiovascular Developmental Biology Center, Medical University of South Carolina. He is involved with The South Carolina Project for Organ Biofabrication, one of the groups at the forefront of 3-D bioprinting research. He explained there are too few organ donors to meet demand and there is an even greater need for soft tissues for reconstructive surgeries for things such as injuries, burns, infections, tumor resections and congenital malformations.
“There are too few organ donors to meet the needs,” Markwald said. “At least 21 people die each day because of the lack of implants.”
This organ shortage might be solved in the future by bioprinting organs on-demand. Biomaterials can be printed using current technology, but there is a fatal flaw. “The Achilles heel of tissue engineering today is the need to create vascularity in the structure, and that has been the focus of what we have been trying to do,” Markwald said.
The key to printing vascularizable micro-organs may involve chemical modifications of alginate hydrogels. Markwald’s lab created an oxidized alginate, which is biodegradable and provides stability for 3-D bioprinting. It also is bioactive, allowing cells to migrate and remodel. They created “plug and play” molds to prepare micro-organ constructs for surgical implantation. These are made with the biodegradable alginate, which contain small molecules to promote host vascular in-growth and suppress inflammatory responses.
Bioprinting is enabled using a “biopaper” made of bioresorbable hydrogels. These allow printing of the cells against gravity and allow the cells to grow, interact and function physiologically. Markwald said research is leading to the development of hydrogels specific to each type of organ tissue.
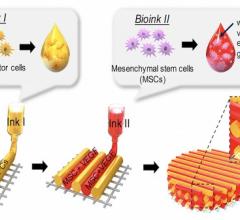
The “bioink” is made from 300 micron diameter spheroids that contain between 8,000-12,000 autologous adipose-derived stem cells. He said it takes about 7 million cells to make 840 spheroids, and it takes thousands of these spheroids to print a 1 mm cube.
Just as 3-D printing allows simultaneous printing of several different colors of materials to build a color 3-D model, bioprinting is being developed to allow use of several different cell types to create complex tissue units.
“Eventually we will be able to make functional hearts or livers,” Markwald said. “What we can print right now are cardiac patches and small- to medium-sized blood vessels, skin tissue, soft tissue (adipose, muscle) for reconstructive surgery, and vascularized micro-organs that can be grown in a bioreactor and used to supplement the function of a diseased organ like the liver.”
Creating 3-D Printable Files
Creating files for 3-D printing from medical imaging datasets starts with good imaging, said Shuai Leng, Ph.D., associate professor of medical physics, Mayo Clinic, Rochester, Minn. “If you start with garbage in, you get garbage out, so you need good image quality,” he stressed.
To create a usable 3-D file, he suggests using 0.6 mm thin imaging slices. This allows for very smooth surfaces. By comparison, he said use of 6 mm slices will make the printed object very rough and textured, appearing pixelated, when it is printed in 3-D.
He said dual-energy CT is great for 3-D printing because it can easily exclude bone so only blood vessels or soft tissue remain in the image area. Metal implants commonly cause problems when creating 3-D printing files, but dual-energy systems have metal artifact reduction software to separate the metal and artifacts from the anatomy to allow creation of better models.
When using 3-D models for procedural planning and navigation, you need to ensure the precision of the model by using U.S. Food and Drug Administration (FDA)-cleared 3-D printing software. The resulting printed models also should be compared to the original images to ensure quality control. Before printing, images should be checked in three planes and approved by a radiologist or the ordering physician.
The final imaging files are converted into STL/CAD files that can be read by the 3-D printers and translated into the final 3-D object.
Legal Considerations Regarding 3-D printing
The field of 3-D printing comes with a new set of legal questions hospitals using the technology will need to consider, said Bruce Kline, a technology licensing manager who oversees patents for new technology developed at Mayo Clinic. For starters, he said the STL file printers use are a lot like MP3 music files, in that they can be protected under copyright and require licensing to use. Copyright violations can occur if a purchased STL anatomical model file for rare disease is illegally shared with another institution that did not purchase the file from the vendor that created the file.
Under the law, if a device has a functional use it falls under patent law. If it is not functional, it falls under copyright law. Kline said most medical 3-D printing for educational models and complex anatomy evaluation currently falls under copyright. But, he said that will rapidly change in the coming years as customizable 3-D printable medical devices see wider use.
Additive manufacturing allows the creation of patient-specific devices at the point of care. Kline said an interesting fact is that these devices are FDA 510(k)-exempt if produced by a hospital instead of a medical device vendor. He said this blurs the lines between traditional vendor relationships, since the hospital can now become the manufacturer. However, if a hospital makes a device, it also becomes liable for it. He advised that it might be better for a commercial vendor to make the device for the hospital so the vendor assumes the liability of the device.
Custom-made medical devices are also exempt under FDA regulations, Kline said. So, if a physician creates or modifies a device to meet the clinical needs of a specific patient's anatomy, he said it is acceptable to use under current FDA rules. This may leave the door wide open for use of 3-D printed devices that are customized for each patient using their own 3-D imaging datasets.
Read the article “FDA Changes Rules For Custom Medical Device Exemptions” to find out how this may impact 3-D printed medical devices.
It is possible printable device files may become available in the next few years to customize and print on demand. However, Kline said it will be much more difficult to enforce patents on these types of devices. He explained if someone makes one or two devices, there is no economical way for the creator of those device files to go after the user/maker of unlicensed copies of the device to claim lost profits.
Currently, Kline said surgical planning models created with 3-D printing are not reimbursable. No CPT code exists for their use, because he said CPT codes are based on clinical trial data showing clinical efficacy to justify reimbursement.
Proposed FDA Guidance for 3-D Printing
In May, the FDA released the draft guidance “Technical Considerations for Additive Manufactured Devices,” for public comment. It is a leapfrog guidance document to provide FDA's initial thoughts on technical considerations specific to 3-D printed devices. Specifically, this draft guidance outlines technical considerations associated with additive manufacturing processes, and the testing and characterization for final finished devices fabricated using 3-D printing. It is intended to serve as a mechanism by which the agency can share initial thoughts regarding the content of premarket submissions for emerging technologies and new clinical applications that are likely to be of public health importance very early in product development. The draft document was created following a fall 2014 workshop where 3-D printing experts discussed all the facets of 3-D printing and attempted to anticipate the issues and questions that will be raised as 3-D printable devices begin to come before the FDA for review in the coming years.
The FDA notes that in medical device applications, 3-D printing has the advantage of facilitating the creation of anatomically matched devices and surgical instrumentation by using a patient's own medical imaging. The FDA said another advantage is the ease in fabricating complex geometric structures, allowing the creation of engineered open lattice structures, tortuous internal channels and internal support structures that would not be easily made or possible using traditional manufacturing approaches.
However, the FDA stated the unique aspects of the printing process, such as the layer-wise fabrication and the relative lack of history of medical devices manufactured using 3-D printing techniques, pose challenges in determining optimal characterization and assessment methods for the final finished device. There are also questions as to the optimal process validation and verification methods for these devices. The FDA is gathering public feedback on the draft document through August, 2016. The draft document can be found online at www.fda.gov/ucm/groups/fdagov-public/@fdagov-meddev-gen/documents/document/ucm499809.pdf
Partnerships Make 3-D More Accessible
The setup and maintenance costs for 3-D printing are more involved than many hospitals want to get involved with. This is especially true at centers where there is very limited application, and has led to partnerships between advanced imaging vendors and 3-D printer vendors to create contract services for one-off printing projects.
Advanced visualization software company Vital Images announced a partnership with 3-D printer company Stratasys at the Radiological Society of North America (RSNA) 2015 annual meeting. They created the industry’s first print-on-demand service using Vital’s Vitrea advanced visualization software and Stratasys’ 3-D printing services. Vital Images' software takes patient scans and converts them into STL files that can be sent directly to a 3-D printer, improving workflow efficiency and 3-D printing accessibility.
GE Healthcare is working with 3-D printer vendor Materialise to develop a software package that will allow the easy creation of 3-D printable files from GE 3-D ultrasound sound systems. GE hopes to have commercial product launch for this technology later in 2016.
Materialise already offers its Mimics Innovation Suite software to create 3-D printer files from medical imaging. Its latest version includes the ability to create images not only from MRI and CT datasets, but also from fluoroscopic imaging from C-arms. It also includes a virtual X-ray tool to allow engineers to create projects to find the optimal angle for 2-D/3-D registration. This allows for an evaluation of the 3-D position of bones and implants without a post-operative CT or MRI scan. It has an automated heart segmentation tool to easily separate the cardiovascular anatomy for advanced research and analyses. The vendor said on a good quality dataset, segmentation now requires only a few mouse clicks rather than several hours of tedious work.
Related 3-D printing Content:
The Future of 3-D Printing in Medicine
World’s Smallest Intravascular OCT Imaging Device Created Using 3-D Printing
Researchers 3-D Print a Beating Heart From Human Cells
New Technique Allows More Complicated 3-D Bioprinting
The Use of 3-D Printing in Cardiology
Other articles on future technologies in cardiology and healthcare:
Cardiovascular Advances to Watch in the Next Decade
VIDEO: Editor's Choice of Future Healthcare Technologies at HIMSS 2015











 October 21, 2024
October 21, 2024