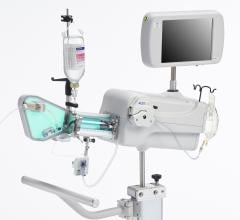
August 6, 2014 — Acist Medical Systems announced it entered into a strategic agreement with Medtronic to co-promote the world’s first Rapid Exchange FFR (RXi) and high definition IVUS (HDi) technologies in the United States. Under this agreement, Acist and Medtronic will work collaboratively to introduce these groundbreaking products into cardiac catheterization laboratories across the United States.
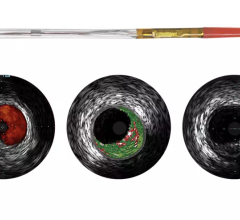
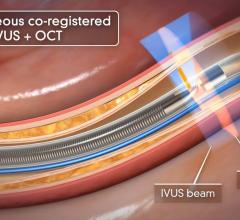
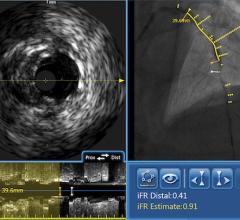
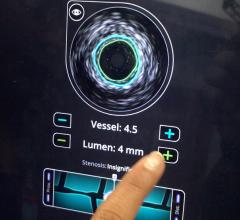
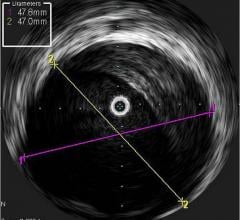
Fractional flow reserve (FFR) has quickly become the standard that interventional cardiologists use to determine which patients can most benefit from stenting. Intravascular ultrasound (IVUS) is an advanced diagnostic modality that provides greater insight into arterial disease than standard angiography alone. The Acist RXi and HDi systems are quicker, easier to use and offer detailed insight that physicians can use to optimize treatment choice for their patients.
Through this collaboration, Acist and Medtronic will bring this technology to existing and new customers who value improved modalities that simplify procedures and benefit their patients.
Acist’s new Rapid FFR system, which utilizes the ultra-thin Acist Navvus Rapid Exchange MicroCatheter and RXi console, was introduced earlier this year in the United States and Europe. The Navvus MicroCatheter can be used over a standard 0.014-inch guidewire, and the RXi system facilitates rapid FFR assessments before, during and post-intervention. This technology is the first of its kind, providing the reassurance of accurate and reliable FFR measurements and the advantages of Rapid Exchange technology.
This agreement makes Acist/Medtronic the forth vendor for FFR and IVUS systems in the U.S. market.
For more information: www.acist.com, www.bracco.com, www.medtronic.com


 September 18, 2025
September 18, 2025