
May 29, 2018 — Results from the innovative SYNTAX III Revolution Trial [1] underline the effectiveness of evolving noninvasive algorithms for fractional flow reserve assessed through computed tomography (FFR-CT) imaging. The data suggests heart teams should use this imaging-based technology for decision making. The data was presented as a late-breaking study at EuroPCR 2018.
“Designed to better understand the heart team decision-making process itself, the SYNTAX III Revolution clinical Trial was distinctive among clinical trials," said Study Chairman Professor Patrick W. Serruys, M.D. "Rather than randomizing the patient, it was the doctors themselves who were randomized — in this case the heart team – in order to understand more completely the present and future role of noninvasive assessments in deciding and planning revascularization strategies.”
The SYNTAX III Revolution trial was specifically designed to provide evidence in decision making by randomizing two heart teams in six participating international centers. These teams, composed of a surgeon, an interventional cardiologist and a radiologist, considered the treatment of 223 patients over an 18-month period.
Each of the two heart teams was presented with the same patient and asked to plan a procedure, answering several questions. These include: Which vessels need to be revascularized? How many bypasses are needed? How many stents should be used? The primary goal was to assess the treatment decision, whether it was decided based on the information received, to go with either surgery (CABG) or PCI.
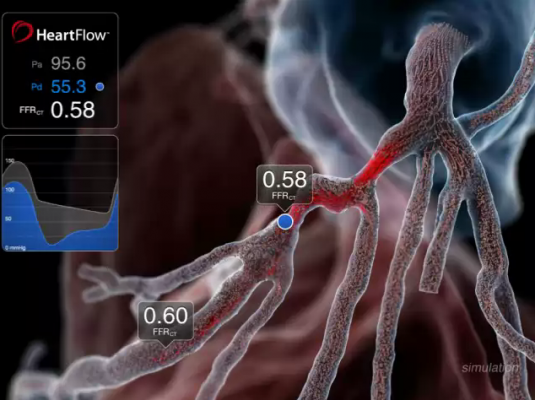
Heart Team A had to make its decision on whether to perform revascularization with either PCI or surgery using information received strictly from noninvasive means using multi-slice coronary computed tomography angiography (MSCT) from a GE Revolution scanner that included CT-FFR assessment using HeartFlow software.
Heart Team B needed to make the same decision but using only conventional, invasive, cine-angiography.
The Cohen’s kappa statistic was employed to indicate the level of agreement between the two heart teams in terms of their treatment decision — in this case to use either CABG or PCI – based on the CT-first assessment or angiography-first evaluation.
“We were looking at the concordance of judgement and the concordance of opinion in the planning of the procedure and discovered that the Cohen’s kappa statistic was very high – 0.82 – which can be called an almost perfect assessment/agreement," Serruys said. He said it showed good agreement as well in terms of the number of bypasses, how many stents should be used and the location in the coronary circulation.
“We were amazed to hear participating surgeons telling us that they could see more things on the GE Revolution multi-slice scan than using conventional angiography,” Serruys continued. “Cine-angiography could be dispensed with completely and only the noninvasive multislice scans used.”
In the initial trial, in order to ensure patient safety, the actual procedure remained “virtual”. After each blinded heart team made its decision and planned for surgery or PCI – and before the actual intervention took place. The different teams were unblinded and provided with all the information acquired by both the MSCT-first assessment and angiography-first examination before actually continuing with the procedure.
In January 2018, preparing for the next step, a meeting was organized with the surgeons of the six centers where they were presented with randomized cases and asked if they could plan an operation using the GE multislice scan alone. “To our surprise,” Serruys said, “there was a very high feeling of feasibility among the surgeons.” The next phase, planned for 2018-2019, will be a first-in-man trial asking surgeons to treat 100 patients based on multislice CT scan alone without looking at coronary angiography.
“In the next five to 10 years, with its increasing accuracy, I think we are going to see the new generation of multislice CT scans replacing conventional cine-angiography," Serruys explained. "For interventional cardiologists this is a bonus, allowing them to have the image before bringing the patient to the cath lab and being able to make a decision concerning whether it is a one-vessel or multivessel disease. If it is clearly one-vessel disease, there is no reason to consider surgery, but, when you know in advance that it is a multivessel disease, you can immediately discuss with the surgeon based on the non-invasive imaging alone and go immediately to the operating room or the interventional suite to perform the intervention, So, this can be seen as promising a real change in our practice. It will take time and it will take multiple trials, but the impetus is there to go in this direction. It is clearly the beginning of a trend we will see in the next few years."
The SYNTAX III trial is an investigator-driven study sponsored by the European Cardiovascular Research Institute (ECRI). For this study, the ECRI received research grants from GE Healthcare and HeartFlow Inc.
Links to other 2018 EuroPCR late-breaking trials
Related FFR-CT Content:
Clinical Applications of FFR-CT
VIDEO: The Status FFR-CT Adoption in the United States
New Technology Supports CT as Prime Cardiac Imaging Modality
Reference:


 February 02, 2026
February 02, 2026 









