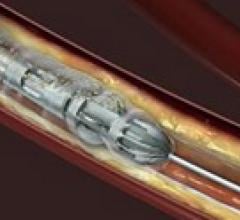
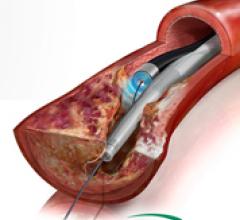
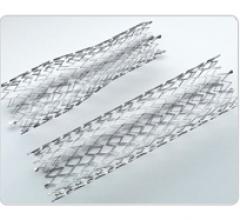
October 17, 2011 — The Detroit Medical Center (DMC) Cardiovascular Institute (CVI) has become the first cardiac care ...
Atherectomy Devices
This channel includes news and new technology innovations for atherectomy systems used in peripheral or coronary arteries to debulk lesions and vessel preparation prior to angioiplasty or stenting.
September 1, 2011 – Medrad Inc. has acquired Pathway Medical Technologies Inc. to strength its vascular interventional ...
As part of its efforts to expand integration of intravascular ultrasound (IVUS) image guidance with cath lab treatment ...
May 5, 2011 – Volcano Corp. announced a supply agreement with ev3, a Covidien company, under which Volcano will supply ...
November 5, 2010 – Cardiovascular Systems Inc. (CSI) will pay $1 million to settle an employment lawsuit with ev3 ...
September 7, 2010 – Enrollment started in the Endovascular Atherectomy Safety and Effectiveness (EASE) study to ...
August 4, 2010 — A second-generation of the Diamondback Predator 360 peripheral arterial disease (PAD) system ...
June 22, 2010 – The U.S. Food and Drug Administration (FDA) recently granted 510(k) clearance for the Jetstream G3 ...
June 11, 2010 – A multicenter, randomized trial planned in the United States will compare laser ablation followed ...
June 2, 2010 – Covidien announced yesterday it is purchasing ev3 Inc. for $2.6 billion to expand its percutaneous ...
May 27, 2010 – The first patient was recently enrolled in the ORBIT II investigational device exemption (IDE) ...

Atherectomy is a key treatment option in peripheral artery disease (PAD), and several physicians Diagnostic & ...

In recent years, many medical device companies saw the underserved lower extremity as an opportunity to enter the ...
April 28. 2010 – A trial examining the use of an orbital atherectomy device on calcified coronary lesions was ...
March 12, 2010 – A rotational thrombectomy system has been indicated for mechanical declotting of occluded native ...


 October 17, 2011
October 17, 2011