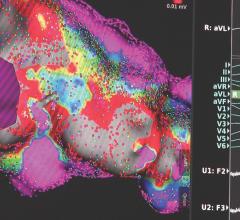
Henry Ford Hospital in Detroit gets a lot of referrals for very sick patients seeking a last resort treatment in its ...
Atherectomy Devices
This channel includes news and new technology innovations for atherectomy systems used in peripheral or coronary arteries to debulk lesions and vessel preparation prior to angioiplasty or stenting.
September 13, 2019 — Rex Medical L.P. has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for ...
August 6, 2019 — Cardiovascular Systems Inc. (CSI) has acquired the Wirion Embolic Protection System and related assets ...

Over the last 40 years, despite multiple advancements in percutaneous coronary interventions, calcified lesions remain a ...
July 18, 2018 — Avinger Inc. announced in June that several physicians have successfully treated over 40 patients for pe ...
May 24, 2018 — Avinger Inc. announced that the company received 510(k) clearance from the U.S. Food & Drug ...
April 4, 2018 — Boston Scientific Corp. announced the acquisition of Securus Medical Group Inc., a privately-held ...
January 16, 2018 — Medtronic plc announced the company’s Neurovascular business unit received U.S. Food and Drug ...
January 9, 2018 – Avinger Inc. announced that Arne Schwindt, M.D., a vascular surgeon at St. Franziskus Hospital in ...

As debates about the current state and future of healthcare rage in Congress, the media and healthcare settings across ...
November 20, 2017 — Avinger Inc. announced in October that the company received 510(k) clearance from the U.S. Food and ...
DAIC Editor Dave Fornell shows some of the innovations displayed on the expo floor at the 2017 Transcatheter ...
September 29, 2017 — Avinger Inc. recently announced Conformité Européenne (CE) Marking approval for treating in-stent ...
September 20, 2017 — Not-for-profit preclinical research institute CBSET announced that its scientists have published ...
September 13, 2017 — Philips announced its presence at the Vascular Interventional Advances (VIVA 17) Annual Conference ...


 October 08, 2019
October 08, 2019