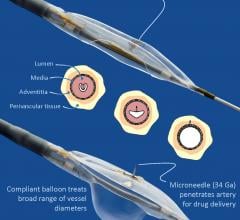
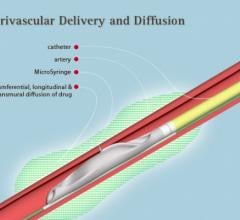
August 9, 2017 — Ra Medical Systems announced that the United States Patent and Trademark Office (USPTO) has granted the ...
Atherectomy Devices
This channel includes news and new technology innovations for atherectomy systems used in peripheral or coronary arteries to debulk lesions and vessel preparation prior to angioiplasty or stenting.
May 30, 2017 — The U.S. Food and Drug Administration (FDA) announced that it has granted market clearance to Ra Medical ...
April 27, 2017 — Mercator MedSystems Inc. announced the first patient enrollment into the TANGO (Temsirolimus ...
April 19, 2017 — Cardiovascular Systems Inc. (CSI) announced April 18 it had initiated a voluntary recall of its 7-10014 ...
April 4, 2017 — Cardiovascular Systems Inc., in partnership with the Cardiovascular Research Foundation (CRF), announced ...
February 8, 2017 — Cardiovascular Systems (CSI) presented six-month data from its LIBERTY 360° post-market study in a ...
February 3, 2017 — Avinger Inc. recently announced positive two-year clinical data from the pivotal VISION study of the ...
December 23, 2016 — The U.S. Food and Drug Administration (FDA) granted a new 510(k) clearance for Avinger’s Lightbox ...
October 24, 2016 — Medtronic received U.S. Food and Drug Administration (FDA) 510(k) clearance for the HawkOne ...
October 20, 2016 — Avinger Inc. recently announced that the company has received expanded indications from the U.S. Food ...
October 7, 2016 — Mercator MedSystems recently announced that 13-month data from the DANCE trial was presented during a ...

Due to poor outcomes from percutaneous transluminal angioplasty (PTA) ballooning of vessels alone, or of stenting in ...
August 16, 2016 — Cardiovascular Systems Inc. (CSI) released procedural and 30-day results from its LIBERTY 360° study ...
May 6, 2016 — Cardiovascular Systems Inc. shared three-year results from its pivotal ORBIT II study in a featured ...
March 3, 2016 — Avinger Inc. announced the company has received 510(k) clearance from the U.S. Food and Drug ...


 August 09, 2017
August 09, 2017