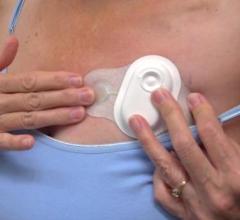
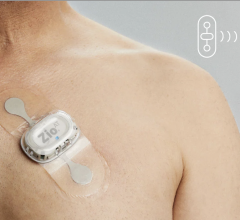
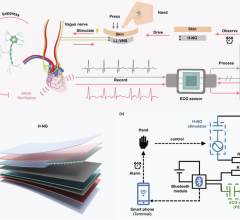
When the patients of Michael Boler, M.D. need cardiac monitoring, the Holter monitor is no longer his first choice. “The ...
Atrial Fibrillation
This channel includes news and new technology innovations for the treatment of atrial fibrillation, also referred to as AF or afib. AF is a cardiac arrhythmia caused by irregular and often rapid heart rate. It is caused by the upper chambers (the atria) beating irregularly and uncoordinated with the lower ventricle chambers of of the heart. Symptoms include weakness with heart palpitations and shortness of breath. The conditional can lead to an increased risk of stroke and heart failure. AF episodes can cause the blood in the atria to stagnate and form clots, usually within the left atrial appendage (LAA). The clots can flow to the brain and cause a stroke. Treatments include anticoagulation therapy to dissolve clots, catheter or surgical ablation and LAA occlusion.
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...
Improved short-term monitoring methods for patients with stroke risk can increase early detection of atrial fibrillation ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
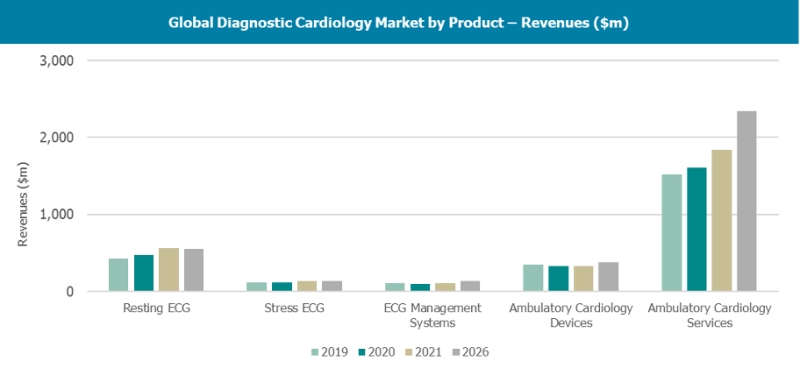
Like most healthcare markets, the diagnostic cardiology market has had a bumpy ride in recent years. The COVID-19 pandem ...
August 17, 2022 — A new research paper was published in Aging (“Aging (Albany NY)” by Medline/PubMed, “Aging-US” by Web ...
July 29, 2022 — A cohort study of persons with incident atrial fibrillation (AF) has found that AF after noncardiac ...
For over a decade, the cardiac cryoablation industry has seen little in the way of technological advancements. Yet ...
July 25, 2022 — iRhythm Technologies, Inc., a leading digital healthcare solutions company focused on the advancement ...
July 22, 2022 — BioSig Technologies, Inc. a medical technology company advancing electrophysiology workflow by ...
July 20, 2022 — CardioFocus, Inc., a medical device company dedicated to advancing ablation treatments for atrial ...
In the United States, the options currently available for cardiac ablation use thermal mechanisms to ablate tissue and ...
July 11, 2022 — BioSig Technologies, Inc., a medical technology company advancing electrophysiology workflow by ...
July 6, 2022 — Atrial fibrillation (AF) is a prevalent cardiovascular condition with one of the highest rates of ...
June 30, 2022 — A new onset of a rapid or irregular heartbeat that develops after surgery, often within a few days, is ...
When the patients of Michael Boler, M.D. need cardiac monitoring, the Holter monitor is no longer his first choice. “The ...
May 24, 2022 — Attune Medical, a pioneering medical device company with a technology platform based on temperature ...
May 18, 2022 — Continuous monitoring with an insertable cardiac monitor (ICM) is shown to be more successful in ...
May 17, 2022 — Results of a new study reveal Black patients hospitalized with atrial fibrillation (AF) are under ...


 August 24, 2022
August 24, 2022