Dec. 3, 2025 — Atraverse Medical, a medical device company developing next-generation left-heart access technology, has ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
Dec. 3, 2025 — Recross Cardio Inc., a structural heart company developing next-generation membrane sealing technology ...
Dec. 2, 2025 — At RSNA 2025, Siemens Healthineers announced Syngo.CT Coronary Cockpit1, a new software solution within ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
Nov. 24, 2025 — InspireMD, Inc., developer of the CGuard Prime carotid stent system for the prevention of stroke ...
Nov. 11, 2025 -— Integra LifeSciences Holdings Corp. has announced the FDA 510(k) clearance for use of its CUSA Clarity ...
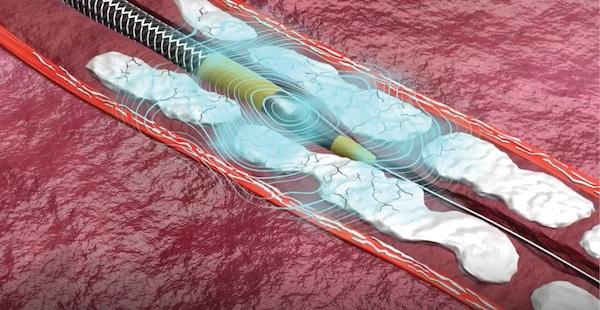
Since receiving FDA approval in 2016, intravascular lithotripsy (IVL) systems have grown in popularity among ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
Nov. 11, 2025 — FastWave Medical has successfully completed enrollment in its 30-patient coronary feasibility study and ...
Nov. 10, 2025 — Sentante, a med-tech robotics company, has successfully demonstrated a first-of-a-kind remote stroke ...
Nov. 4, 2025 — Amplitude Vascular Systems (AVS), a medical device company focused on treating calcified arterial disease ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
Nov. 3, 2025 — Penumbra, Inc. has announced additional results of the STORM-PE randomized controlled trial (RCT), which ...
Nov. 4, 2025 – Johnson & Johnson MedTech has announced the one-year results in patients treated with its Shockwave ...
Oct. 28, 2025 — Results from the first-of-its-kind randomized PROCTOR trial found that a strategy of saphenous vein ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
Oct. 30, 2025 — Recor Medical and its parent company, Otsuka Medical Devices presented the results from two clinical ...
Oct. 22, 2025 -- Medical robotics innovator Noah Medical presented two new data sets highlighting the clinical and ...
Oct. 29, 2025 — FastWave Medical presented new first-in-human (FIH) and pre-clinical data for its Sola coronary laser ...


 December 05, 2025
December 05, 2025