June 20, 2013 — Biosensors International Group Ltd. has entered into a licensing agreement with Eurocor GmbH, a group ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
June 14, 2013 — Criteria for evaluating operator competency for performing coronary interventions should be expanded ...

June 14, 2013 — Preliminary results from the ADVISE (Adenosine Vasodilator Independent Stenosis Evaluation) II trial ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
June 13, 2013 — St. Jude Medical Inc. announced U.S. Food and Drug Administration (FDA) approval to begin the EnligHTN ...
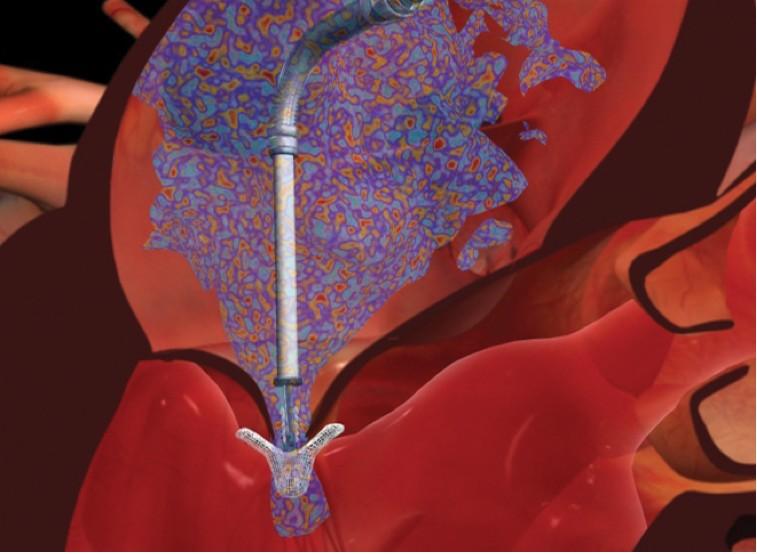
June 13, 2013 — Abbott announced publication of positive outcomes from two European post-approval studies of the ...
June 13, 2013 — Tryton Medical Inc. announced that the Nordic-Baltic Bifurcation Study Group will investigate the Tryton ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
The phrase “doing more with less” is becoming more prevalent in tightening economic environments, and the operating room ...
June 11, 2013 — New long-term data from the DIVERGE clinical study, presented at EuroPCR 2013 by Principal Investigator ...

June 10, 2013 — Direct Flow Medical Inc. announced that it has received approval from the U.S. Food and Drug ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
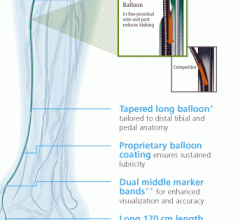
June 10, 2013 — C. R. Bard Inc. announced the enrollment of the first patient into the Lutonix Below the Knee (BTK) Clin ...
June 7, 2013 — AngioLight has successfully completed an initial animal study of the AngioLight System at Fu Wai Hospital ...
June 6, 2013 — Boston Scientific Corporation reported interim data from the REDUCE-HTN clinical program, which ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
June 4, 2013 — Covidien recently introduced its RapidCross 0.014-inch Rapid Exchange Percutaneous Transluminal ...
June 3, 2013 — St. Jude Medical Inc. announced that the company’s EnligHTN multi-electrode renal denervation system ...

June 3, 2013 — Medtronic Inc. announced it has received CE mark for valve-in-valve (VIV) procedures using the CoreValve ...

 June 20, 2013
June 20, 2013