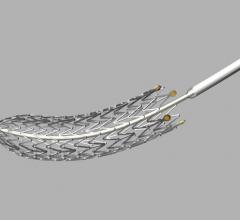
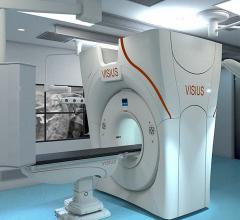
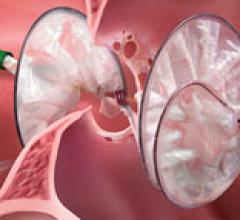
August 19, 2013 — Medtronic Inc. announced the submission of its first pre-market approval (PMA) module to the U.S. Food ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
August 19, 2013 — Cook Medical is again shipping its Zilver PTX drug-eluting peripheral stent to medical centers in the ...
August 16, 2013 — Patient enrollment has been initiated in a post-market registry for the Combo Dual Therapy Stent to ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
August 15, 2013 — Former President George W. Bush underwent heart surgery on the morning of Aug. 6, 2013, to receive a s ...
August 13, 2013 — IMRIS Inc. has obtained regulatory CE mark for Visius iCT, the first and only ceiling-mounted ...
August 13, 2013 — The William Stamps Farish Fund in Houston has donated $400,000 to a collaboration between the ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
August 8, 2013 — The U.S. Food and Drug Administration (FDA) accepted Biomedica’s Investigational New Drug (IND) ...
August 7, 2013 — W. L. Gore & Associates announced a favorable ruling involving the Gore Helex Septal Occluder. The Hon ...
August 6, 2013 — Vanderbilt Heart and Vascular Institute is participating in the VELOCITY study, a randomized controlled ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
August 5, 2013 — AstraZeneca announced results from a new sub-analysis of the PLATO study that evaluated the incidence ...
August 5, 2013 — Abiomed Inc. reported that physicians have implanted more than 15,000 Impella pumps in U.S. patients ...
August 2, 2013 — The U.S. Food and Drug Administration (FDA) recently granted market clearance for a hand lotion ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
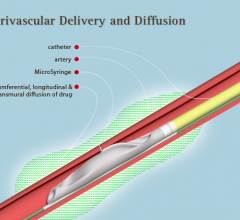
August 2, 2013 — Mercator MedSystems has been issued U.S. Patent 8,465,752, which protects the company’s sole U.S ...
August 1, 2013 — InspireMD Inc. said the first patient has been enrolled in the Master II IDE clinical trial to evaluate ...
The Mikro-Cath disposable blood pressure catheter delivers accurate and reliable hemodynamic data from within the heart ...


 August 19, 2013
August 19, 2013