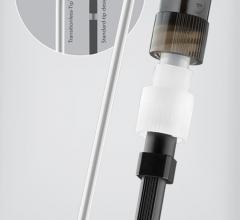
Aug. 8, 2012 — Vascular Solutions Inc. launched the SuperCross FT, a new flexible-tip version of its line of SuperCross ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
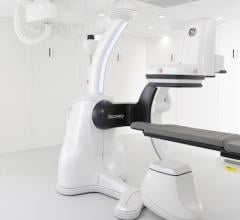
August 3, 2012 — GE Healthcare announced it has already received six orders for its Discovery IGS 730 system since ...

Interventional procedures are growing rapidly and becoming more specialized in diverse areas of care. In the next ...
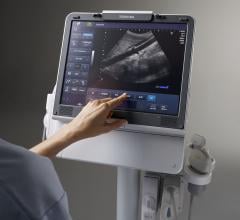
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
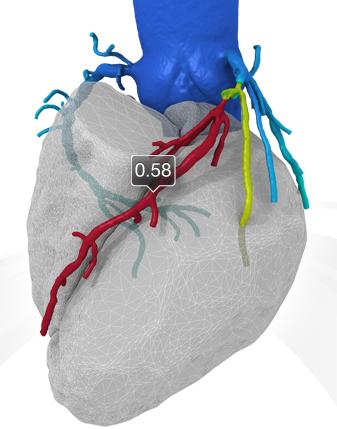
Trends and new technology for cardiac computed tomography angiography (CCTA) were highlighted during the 2012 Society of ...
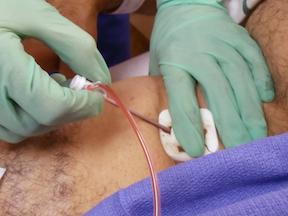
Percutaneous coronary interventional (PCI) procedures are performed throughout hundreds of US institutions every day ...
July 27, 2012 — Cook Medical announced general availability of the Aprima Access nonvascular introducer set, the first ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
July 27, 2012 — C. R. Bard Inc. announced that its Lutonix technology center has completed patient enrollment into its ...

July 27, 2012 – The current Medicare payment system is irreparably flawed and a new, more permanent solution must be ...
July 26, 2012 — Any exposure to radiation, no matter how small, has the potential of leading to future health issues ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
July 26, 2012 — Angion Biomedica Corp. announced that the first patient was dosed in a Phase II multicenter clinical ...

July 25, 2012 — Corindus Vascular Robotics today announced U.S. Food and Drug Administration (FDA) 510(k) clearance for ...
This video, provided by Crux Biomedical, demonstrates the implantation of the FDA-cleared Crux VCF inferior vena cava ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
July 24, 2012 — Use of the retrograde approach to revascularizing coronary chronic total occlusions (CTOs) to deliver ...
July 24, 2012 — The importance of utilizing computed tomography (CT) in pre-planning for transcatheter aortic valve ...
July 20, 2012 — Ticagrelor (Brilinta), a blood-thinning drug approved by the U.S. Food and Drug Administration (FDA) in ...

 August 08, 2012
August 08, 2012