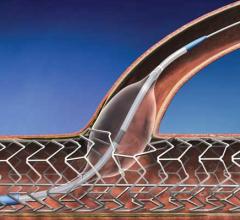
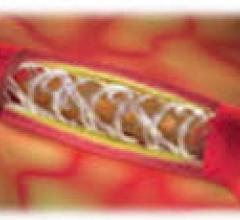
July 20, 2012 — The ability to see inside the arteries of vascular disease patients in high resolution before and during ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
July 20, 2012 — Crux Biomedical announced it has received U.S. Food and Drug Administration (FDA) clearance for its ...
July 20, 2012 — Sony Electronics is announcing the world's first medical-grade monitor, model PVM-2551MD, based on ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...

Each year, I find it interesting that some “new” technology being introduced by a vendor is actually an old, recycled ...

Atherectomy devices are used in the cath lab to debulk lesions by cutting or laser ablating plaque, calcium and tissue ...

There is much discussion about the possibility of using transradial artery access to help reduce door-to-balloon times ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...

Cath lab volumes have gone down in recent years for several reasons, but one of the biggest reasons might be market ...
July 17, 2012 — Panasonic announced a new 3-D medical-grade 32-inch class monitor, the EJ-MDA32U-K. The new 3-D monitor ...
July 17, 2012 — Arterial Remodeling Technologies (ART) reported that they have achieved a medical milestone with its ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...

As the average age of the U.S. population and the number of patients at high risk for cardiovascular disease ...
July 16, 2012 — TriReme Medical Inc. (TMI) announced that it has received U.S. Food and Drug Administration (FDA) ...
July 16, 2012 — Mercator MedSystems, a medical technology company whose platform Micro-Infusion Catheter technology ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
July 16, 2012 — Reva Medical Inc. announced it completed clinical enrollment with the ReZolve drug-eluting bioresorbable ...
July 16, 2012 — The U.S. Food and Drug Administration (FDA) notified healthcare professionals of a Class I recall of ...
July 13, 2012 — Angioslide Ltd., a provider of embolic capture angioplasty solutions, today announced the successful ...

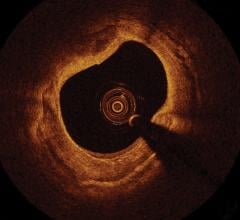
 July 20, 2012
July 20, 2012