March 1, 2019 — iSchemaView announced the release of RAPID Angio, a complete neuroimaging solution for the angiography s ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).

March 1, 2019 — When it comes to treatment options for a damaged heart valve, nothing is getting more attention than tra ...
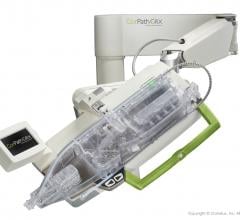
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
February 27, 2019 — Ra Medical Systems Inc. announced a 98 percent success rate in the results from a 52-patient study u ...
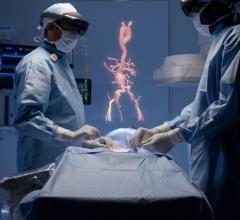
February 25, 2019 — Philips will unveil a new mixed reality concept developed together with Microsoft that the company ...

The Society for Cardiovascular Angiography and Interventions (SCAI) 2019 Scientific Sessions in Las Vegas, May 19-22 ...
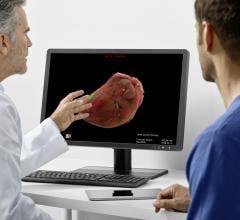
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...

February 22, 2019 — The U.S. Food and Drug Administration (FDA) has approved the Biotronik Orsiro drug-eluting stent ...
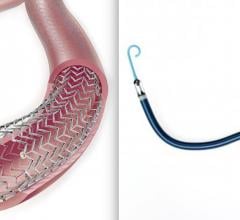
February 21, 2019 — Navitian, the new coronary microcatheter from iVascular, recently received CE mark approval. The ...
Clinical study data makes the world go around in cardiology and is the basis of setting guidelines in evidence-based ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
February 20, 2019 — California-based Vascular Dynamics Inc. (VDI) is sponsoring a new clinical trial, called CALM-2 ...
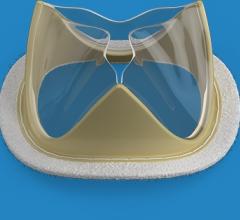
February 18, 2019 — Foldax Inc. announced the U.S. Food and Drug Administration (FDA) has granted investigative device ...
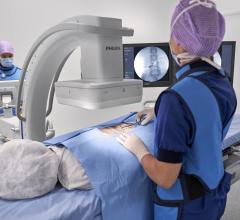
February 18, 2019 — Philips announced the launch of Philips Zenition, its new mobile C-arm imaging platform. Mobile C ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
February 15, 2019 — Corindus Vascular Robotics Inc. is seeking premarket clearance from the U.S. Food and Drug ...
February 14, 2019 — Abdominal aortic stent grafts will remain the largest segment in the global aortic stent graft ...
February 13, 2019 — At the 2019 Healthcare Information and Management Systems Society (HIMSS) global conference and ...

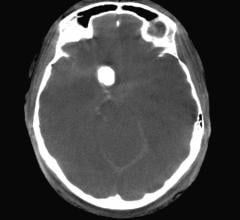
 March 01, 2019
March 01, 2019