June 9, 2014 — At the Society of Nuclear Medicine and Molecular Imaging (SNMMI) Annual Meeting, GE Healthcare introduced ...
Diagnostic and Interventional Cardiology DAIC
Diagnostic and Interventional Cardiology (DAIC) is a magazine that reaches more than 25,000 healthcare professionals in cardiology, interventional cardiology and cath labs across the United States. These influential buying team members rely on DAIC's award-winning editorial content and comparison charts as a unique research tool for specifying, recommending and approving technology/device purchases.
June 6, 2014 — Continuing to expand its portfolio of endovascular solutions, Medtronic Inc. announced that two recently ...
June 5, 2014 — Lumedx Corp. and Scientific Software Solutions Inc. (SSS), maker of PedCath congenital catheterization ...

June 5, 2014 — The president of the American College of Cardiology (ACC), Patrick O’Gara, M.D., recently explored what ...
June 5, 2014 — Cook Medical is engaged in two clinical studies that will provide additional data on the safety and ...
June 3, 2014 — Edwards Lifesciences Corp. received CE mark for the advanced Edwards Intuity Elite valve system. The next ...
June 3, 2014 — JenaValve Technology Inc., a privately held, venture-backed developer of transcatheter aortic valve ...

June 2, 2014 — St. Jude Medical announced the completion of its acquisition of the privately held CardioMEMS Inc ...
June 2, 2014 — As more endovascular medical programs adapt simulation as an essential part of their training curriculum ...
June 2, 2014 — June St. Jude Medical Inc. announced preliminary results from the EnligHTN III clinical study, which ...
June 2, 2014 — MedInformatix announced at the annual meeting of the Society for Imaging Informatics in Medicine (SIIM) ...
May 30, 2014 — Direct Flow Medical has received investigational device exemption (IDE) approval from the U.S. Food and ...
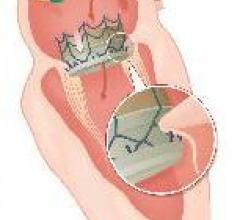
May 30, 2014 — CardiAQ Valve Technologies announced it successfully implanted its second-generation transcatheter mitral ...
May 30, 2014 — The U.S. Food and Drug Administration (FDA) granted market clearance to the CardioMEMS Heart Failure ...
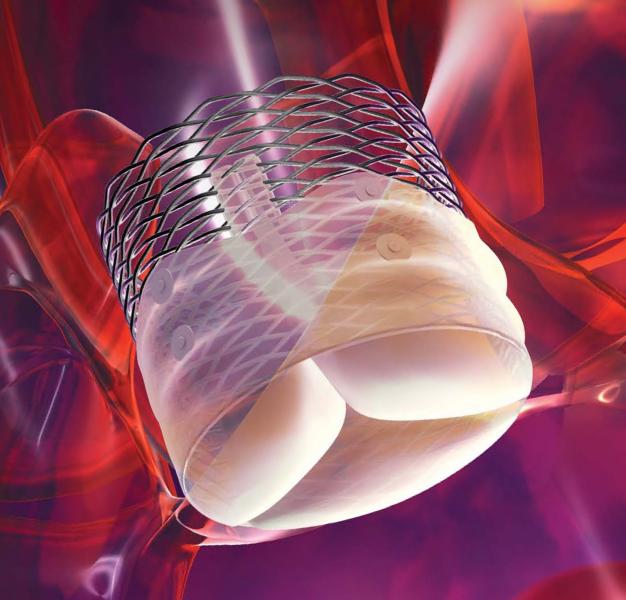
May 30, 2014 — Further validating its advanced transcatheter aortic valve implantation (TAVI) technology, the Boston ...

 June 09, 2014
June 09, 2014