May 19, 2014 — Six independent studies published within the past 10 years have shown a link between whole body vibration ...
Diagnostic and Interventional Cardiology DAIC
Diagnostic and Interventional Cardiology (DAIC) is a magazine that reaches more than 25,000 healthcare professionals in cardiology, interventional cardiology and cath labs across the United States. These influential buying team members rely on DAIC's award-winning editorial content and comparison charts as a unique research tool for specifying, recommending and approving technology/device purchases.
May 19, 2014 — For the first time, a minimally invasive transcatheter valve — tested by Baylor Research Institute in ...

May 16, 2014 — Fetal heart experts working with the American Heart Assn. have developed guidelines to help healthcare ...
May 15, 2014 — Medweb announced the introduction of several new features for Medweb Collage that will further enable the ...
May 15, 2014 — Blackford Analysis is demonstrating its suite at the 2014 annual meeting of the Society for Imaging ...
May 15, 2014 — Digisonics (Booth #211) will showcase its Enterprise PACS and Structured Reporting Solutions for all ...
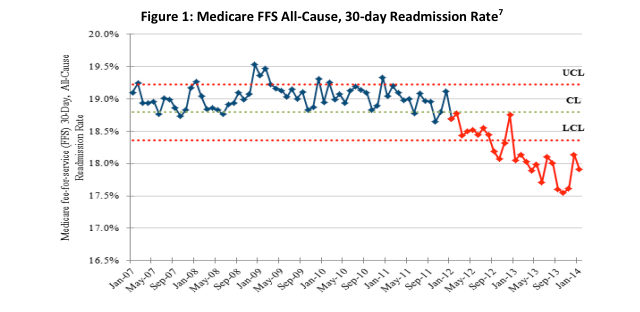
May 14, 2014 — The Department of Health and Human Services (HHS) announced that new preliminary data show an overall 9 ...

May 14, 2014 — Data presented during Heart Rhythm 2014 found the use of quadripolar leads reduced the number of ...
May 13, 2014 — Esaote North America received U.S. Food and Drug Administration (FDA) clearance to market and sell its ...
May 12, 2014 — Datascope Corp./Maquet initiated a voluntary worldwide field correction of some of its intra-aortic ...

May 9, 2013 — Medical device executives from across the region are not sure the influx of new patients using their ...
May 8, 2014 — Abbott announced its catheter-based MitraClip therapy has received Health Canada approval, providing ...

May 8, 2014 — The past year has been a turbulent one for U.S. physicians, according to the 2014 Practice Profitability ...
May 7, 2014 — HeartWare Intl. Inc. issued a voluntary Urgent Medical Device Correction related to all HeartWare ...
May 7, 2014 — GE Healthcare received U.S. Food and Drug Administration (FDA) clearance for its Discovery IGS 740, a new ...


 May 19, 2014
May 19, 2014