There is a growing trend in the use of small, portable extracorporeal membrane oxygenation (ECMO) systems for ...
Diagnostic and Interventional Cardiology DAIC
Diagnostic and Interventional Cardiology (DAIC) is a magazine that reaches more than 25,000 healthcare professionals in cardiology, interventional cardiology and cath labs across the United States. These influential buying team members rely on DAIC's award-winning editorial content and comparison charts as a unique research tool for specifying, recommending and approving technology/device purchases.
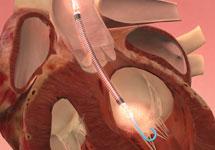
Since the 1970s, intra-aortic balloon pumps (IABPs) have been the gold standard of minimally invasive hemodynamic ...
May 13, 2013 — Live demonstration, transmission or recording of transcatheter aortic valve replacement (TAVR) has been ...

May 13, 2013 — Boston Scientific reports that the four-year follow-up data from the PROTECT AF clinical trial demonstrat ...
May 2, 2013 — Simbionix USA Corp. released various training modules for the Angio Mentor endovascular simulator.
The ...
May 1, 2013 — Zoll Medical said results from the prospective registry and follow-up of Patients Using the Wearable Defib ...
May 1, 2013 — InterValve Inc. announced that it has received 510(k) clearance to market the new V8 Aortic Valvuloplasty ...
April 30, 2013 — Philips Healthcare has completed patient enrollment in the CHILL-MI clinical study designed to further ...
April 29, 2013 — Cook Medical has launched a new fully-retractable .035 inch embolization coil, intended for peripheral ...
April 25, 2013 — Stenting reopens completely blocked bowel arteries, preventing damage and even death from a condition ...
April 24, 2013 — Cardiosonic Inc. announced the completion of the first phase of patient enrollment in its first-in-man ...
April 22, 2013 — Zoll Medical Corp. has entered into an agreement with Reflectance Medical Inc. to develop and market a ...
April 18, 2013 — Abbott has received Conformite Europeene (CE) marking for the Architect Galectin-3 assay, a blood test ...
April 17, 2013 — Multi-detector computed tomography (MDCT) is a better way to measure annular size in patients with ...

The staffing firm SpringBoard Healthcare is conducting a wage information survey to gather information about cath lab’s ...


 May 13, 2013
May 13, 2013