August 24, 2011 — The U.S. Department of Health and Human Services (HHS) announced a new initiative to improve care for ...
Diagnostic and Interventional Cardiology DAIC
Diagnostic and Interventional Cardiology (DAIC) is a magazine that reaches more than 25,000 healthcare professionals in cardiology, interventional cardiology and cath labs across the United States. These influential buying team members rely on DAIC's award-winning editorial content and comparison charts as a unique research tool for specifying, recommending and approving technology/device purchases.
August 23, 2011 — Cardium Therapeutics received the final $1.1 million payment from Royal Philips Electronics in ...
August 19, 2011 - Neovasc Inc. accomplished $4.7 million in financing to complete the COSIRA clinical trial for the ...
August 19, 2011 - Endologix Inc. said a ruling this week will likely aid its defense in a stent graft patent litigation ...
August 19, 2011 — NeoStem Inc., an international biopharmaceutical company, reported progress toward commencement of a ...
August 12, 2011 — Endologix Inc. announced that the first American commercial implant of the company's AFX Endovascular ...
August 12, 2011 — SynCardia Systems Inc., manufacturer of the temporary Total Artificial Heart (TAH), said Aug. 2 ...
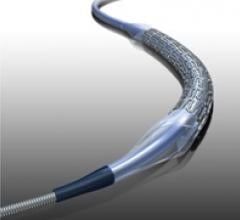
August 10, 2011 – The U.S. Food and Drug Administration (FDA) has approved Abbott’s RX Herculink Elite Renal Stent ...
August 8, 2011 – Edwards Lifesciences announced the global launch of the Carpentier-Edwards Physio Tricuspid ...
August 5, 2011 — Heart failure circulatory support system maker Thoratec Corp. announced it acquired the medical ...
August 5, 2011 — BG Medicine Inc., a company specializing in novel, biomarker-based diagnostics, will present results ...

August 3, 2011 — Texas Children's Hospital (TCH) is the nation's first pediatric hospital to both surgically implant and ...
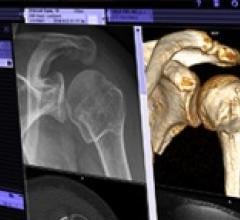
August 3, 2011 — Siemens Healthcare announced that its syngo.via advanced visualization software will be exclusively ...
August 3, 2011 — AmbiCom Holdings Inc., developer of wireless products for medical equipment suppliers, announced that ...
August 2, 2011 – The U.S. Food and Drug Administration said it recently cleared Abbott Vascular’s RX Herculink Elite ...

 August 24, 2011
August 24, 2011