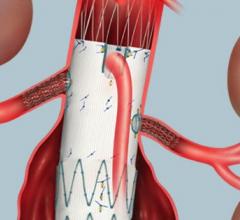
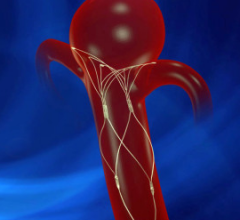
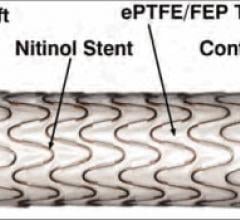
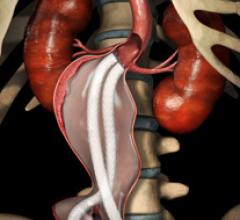
October 3, 2014 — W. L. Gore & Associates Inc. announced that the U.S. Food and Drug Administration (FDA) has approved ...
Endovascular Aortic Repair
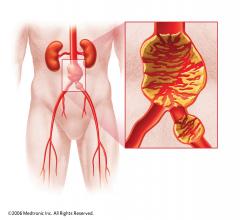
Endovascular aortic repair (EVAR) in a transcatheter method to repair abdominal aortic aneurysms (AAA) and thoracic endovascular aortic aneurysm repair (TEVAR). The procedure usually involves placement of self-expanding,covered stent grafts to close off the aneurysm and prevent further aortic leaks. This can be used instead of open vascular surgery. EVAR today accounts for the majority of aortic aneurysm repairs compared to surgery.
September 15, 2014 — A new minimally invasive surgery involving a stent graft made from a 3-D image of the patient’s ...
September 8, 2014 — The U.S, Food and Drug Administration (FDA) has cleared MicroVention Inc.’s Low-Profile Visualized ...

July 15 2014 — Researchers at the University of California, San Diego School of Medicine have documented the safety ...
June 20, 2014 — Pulsar Vascular announced it received U.S. Food and Drug Administration (FDA) investigative device ...
June 6, 2014 — Continuing to expand its portfolio of endovascular solutions, Medtronic Inc. announced that two recently ...
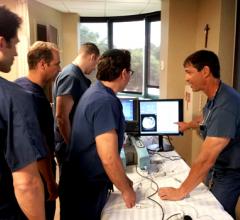
June 2, 2014 — As more endovascular medical programs adapt simulation as an essential part of their training curriculum ...
May 5, 2014 — W. L. Gore & Associates (Gore) announced that Himanshu Patel, M.D. and David Williams, M.D. at The ...
March 6, 2014 — W. L. Gore & Associates’ Gore Excluder Iliac Branch Endoprosthesis is a complete, fully engineered ...
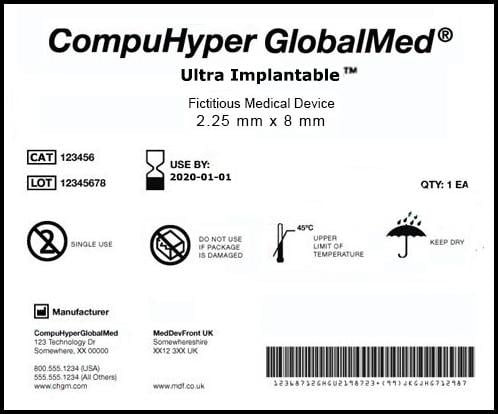
September 25, 2013 – The U.S. Food and Drug Administration (FDA) announced a final rule for the unique device ...

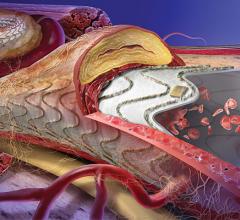
 October 03, 2014
October 03, 2014