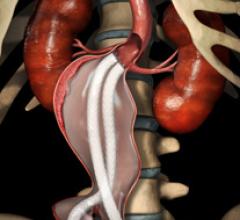
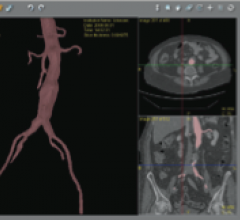
October 4, 2012 — Aptus Endosystems Inc., a medical device company developing advanced technology for endovascular ...
Endovascular Aortic Repair
Endovascular aortic repair (EVAR) in a transcatheter method to repair abdominal aortic aneurysms (AAA) and thoracic endovascular aortic aneurysm repair (TEVAR). The procedure usually involves placement of self-expanding,covered stent grafts to close off the aneurysm and prevent further aortic leaks. This can be used instead of open vascular surgery. EVAR today accounts for the majority of aortic aneurysm repairs compared to surgery.
September 21, 2012 — Endologix Inc., developer and marketer of treatments for aortic disorders, announced earlier this ...
Due to the increasing number of transcatheter aortic valve replacements (TAVR) and endovascular aneurysm repair (EVAR) ...

Cath lab volumes have gone down in recent years for several reasons, but one of the biggest reasons might be market ...
July 9, 2012 — Endosystems Inc., a medical device company developing technology for endovascular aneurysm repair (EVAR) ...
June 22, 2012 –– The U.S. Food and Drug Administration (FDA) recently selected a stent graft being developed by ...
June 8, 2012 — Medtronic Inc. announced the U.S. launch of the Endurant II AAA Stent Graft System, which recently ...
Methodist DeBakey Heart and Vascular Center in Houston, Tex., has leveraged new imaging and interventional technologies ...
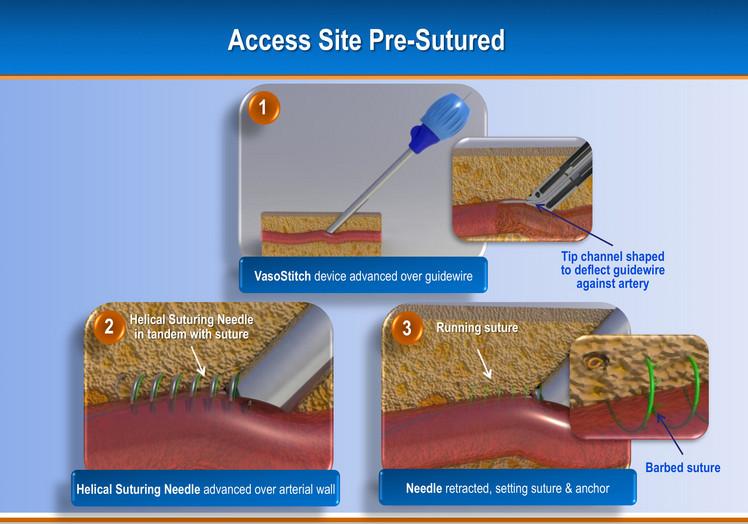
April 20, 2012 — VasoStitch will be featuring its technology at EuroPCR, Europe’s largest interventional cardiology scie ...
February 24, 2012 — Simbionix USA Corp. announced that it received U.S. Food and Drug Administration (FDA) clearance for ...
February 16, 2012 — Aptus Endosystems Inc. a medical device company developing advanced technology for endovascular ...

Based on my observations and those of the doctors on the Diagnostic & Interventional Cardiology Editorial Advisory Board ...
January 18, 2011 – Medtronic Inc. announced the CE mark and international launch of the Endurant II AAA stent graft ...
January 17, 2012 – Terumo Americas Holding Inc., a U.S. subsidiary of Japan's Terumo Corp., announced it acquired Onset ...
January 17, 2012 — The U.S. Food and Drug Administration (FDA) expanded the approved usage for an endovascular graft ...


 October 04, 2012
October 04, 2012