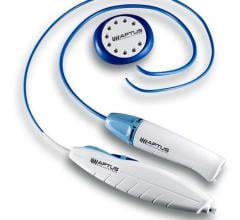
September 18, 2013 – Simbionix USA Corp. released the Endovascular Basic Skills training module for the ANGIO Mentor ...
Endovascular Aortic Repair
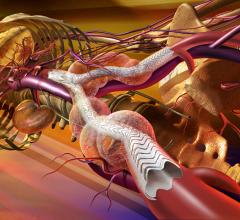
Endovascular aortic repair (EVAR) in a transcatheter method to repair abdominal aortic aneurysms (AAA) and thoracic endovascular aortic aneurysm repair (TEVAR). The procedure usually involves placement of self-expanding,covered stent grafts to close off the aneurysm and prevent further aortic leaks. This can be used instead of open vascular surgery. EVAR today accounts for the majority of aortic aneurysm repairs compared to surgery.
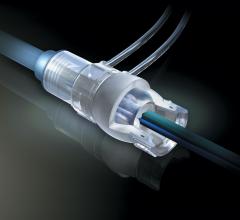
There is no shortage of debate among interventional cardiologists over whether vascular closure devices should be used ...
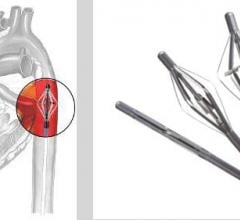
June 24, 2013 — Aptus Endosystems Inc. announced that it received 510(k) clearance from the U.S. Food and Drug ...

May 20, 2013 — Patients with ruptured abdominal aortic aneurysms (AAA) are more than twice as likely to survive if they ...
April 8, 2013 — W. L. Gore & Associates Inc. has received U.S. Food and Drug Administraion (FDA) approval for the new ...
March 27, 2013 — The Heart Failure 2013 LA meeting will include a showcase of several startup companies offering ...
March 25, 2013 — W. L. Gore & Associates introduced its Gore DrySeal Sheath with hydrophilic coating in Europe. The new ...
December 21, 2012 — Despite earlier signs that a less-invasive surgery is safer and better than “open” operations to ...
November 30, 2012 — TeraRecon previewed a flexible new pay-as-you-go billing option for its cloud and laptop users of ...
November 30, 2012 — TriVascular Inc. announced premarket approval (PMA) of the Ovation Abdominal Stent Graft System by ...
Mercy Hospital in Chicago has developed a successful hybrid cath lab program where various specialties work together for ...
November 20, 2012 — W. L. Gore and Associates has announced the commercial availability of its new 10-cm configuration ...
November 19, 2012 — New endovascular grafting techniques to repair tissue following abdominal aortic aneurysm rupture ar ...
November 14, 2012 — Medtronic Inc. announced that the U.S. Food and Drug Administration (FDA) has approved the company’s ...
November 9, 2012 — The U.S. Food and Drug Administration (FDA) recently cleared Bolton Medical’s Relay Thoracic Stent ...


 September 18, 2013
September 18, 2013