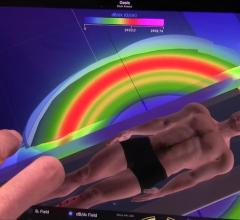
May 24, 2018 — Syncope with bifascicular block may be caused by intermittent complete heart block, but competing ...
Implantable Cardiac Monitor (ICM)
Implantable cardiac monitors (ICM) are small electrophysiology (EP) devices that used for long-term monitoring of a patient's heart electrical activity to detect arrhythmias. The technology can eliminate the need for a bulkly external Holter monitor that requires wire leads attached to the patient. ICMs can be inserted under the skin during and office visit and record cardiac data continuously for up to 4.5 years.
May 9, 2018 — The U.S. Food and Drug Administration (FDA) said it recently granted market clearance for Angel Medical ...
Emanuel Kanal, M.D., director of MRI services and professor of radiology and neuroradiology at the University of ...

November 3, 2017 — Here is the list of the most popular articles and videos on the Diagnostic and Interventional ...
October 23, 2017 – The U.S. Food and Drug Administration (FDA) has cleared Abbott’s Confirm Rx Insertable Cardiac ...
July 14, 2017 — A review appearing in the July 18 issue of the Journal of the American College of Cardiology (JACC) disc ...

Electrophysiology (EP) technology has been advancing rapidly the past few years with new ablation tools to improve ...
May 30, 2017 — Abbott recently announced European CE mark and first use of the new Confirm Rx Insertable Cardiac Monitor ...

May 16, 2017 - The study using small, subcutaneous implantable cardiac monitors for long-term, 24-hour a day monitoring ...
April 19, 2017 — Murj Inc., a digital health company that helps manage implantable cardiac device data, announced more ...
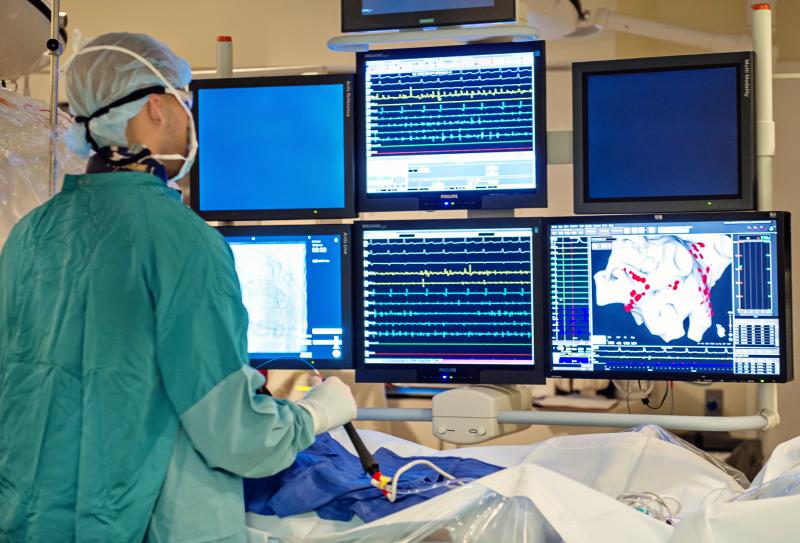
May 16, 2017 — Here is a list of the late-breaking clinical trials presented at the Heart Rhythm Society (HRS) 2017 ...
A discussion with Heart Rhythm Society (HRS) President Michael Gold, M.D., Ph.D., director of cardiology and associate ...
DAIC Editor Dave Fornell takes a tour of some of the interesting new technologies from the vendor booths on the expo ...
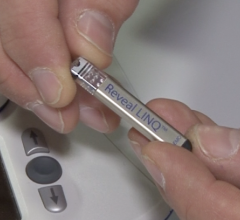
March 15, 2017 — Medtronic plc announced U.S. Food and Drug Administration (FDA) 510(k) clearance for its Reveal LINQ In ...
March 14, 2017 — Climbing above 4,000 meters can provoke abnormal heart rhythms in otherwise healthy mountaineers, with ...

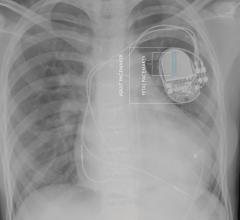
 May 24, 2018
May 24, 2018