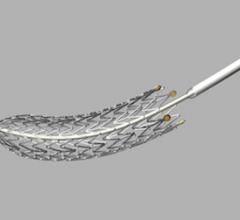
January 31, 2013 — Cardiovascular Systems Inc. (CSI) announced CONFIRM study series data presented at the 2013 ...
Peripheral Artery Disease (PAD)
This channel includes news, interventions, and new technology innovations for peripheral artery diease, PAD and critical limb ischemia.


January 28, 2013 — According to Millennium Research Group (MRG), increasing awareness and diagnosis of peripheral ...
January 28, 2013 — AngioScore Inc. announced the launch of its new 100 mm length AngioSculpt PTA Scoring Balloon ...
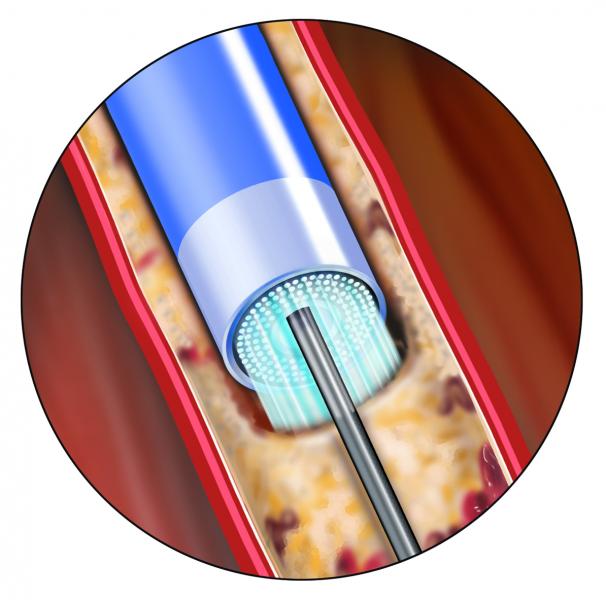
January 24, 2013 — The Spectranetics Corporation announced final results from the PATENT (Photo-Ablation using the TURBO ...
January 22, 2013 — Cordis Corporation announced the presentation of the two-year STROLL clinical study results at the ...

January 16, 2013 — Just weeks after the Food and Drug Administration (FDA) approved Cook Medical’s Zilver PTX drug ...
January 16, 2013 — Biotronik announced the first U.S. implant of their Pulsar-18 self expanding stent in the BIOFLEX-I ...
January 8, 2013 — Angioslide announced the closing of $6.3 million financing led by new investor TriVentures. In ...
January 8, 2013 — Spectranetics Corp. announced an agreement to purchase the assets of Upstream Peripheral Technologies ...
December 26, 2012 — Flexible Stenting Solutions Inc. (FSS) announced it gained CE mark in the European Union for its 6 ...
December 7, 2012 — More than half of patients treated for claudication or critical limb ischemia (CLI) with stenting of ...
Mercy Hospital in Chicago has developed a successful hybrid cath lab program where various specialties work together for ...
November 20, 2012 — Cordis Corp. announced that the U.S. Food and Drug Administration (FDA) has approved the S.M.A.R.T ...
November 16, 2012 – The U.S. Food and Drug Administration (FDA) has granted market clearance for the Avinger Inc. Ocelot ...
November 15, 2012 — The U.S. Food and Drug Administration (FDA) granted market clearance for Cook Medical’s Zilver PTX ...

 January 31, 2013
January 31, 2013