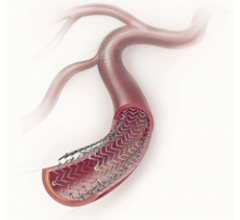
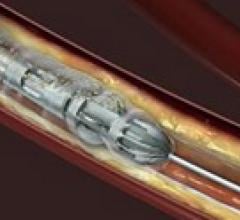
November 14, 2011 – Boston Scientific welcomed positive outcomes from the COBRA clinical trial, which evaluated post ...
Peripheral Artery Disease (PAD)
This channel includes news, interventions, and new technology innovations for peripheral artery diease, PAD and critical limb ischemia.
November 14, 2011 – Medrad Inc. announced that five-year data from the THUNDER trial [1] demonstrated a 59 percent ...
November 4, 2011 — Medtronic Inc. announced approval by the U.S. Food and Drug Administration (FDA) of the Assurant ...
November 1, 2011 – Boston Scientific released the schedule of its major events and product-related clinical research for ...
October 17, 2011 — The Detroit Medical Center (DMC) Cardiovascular Institute (CVI) has become the first cardiac care ...

October 14, 2011 – A U.S. Food and Drug Administration (FDA) review panel gave its unanimous recommendation for the ...
October 3, 2011 – Maquet Cardiovascular has signed a definitive agreement to acquire Atrium Medical Corp. for $680 ...
September 30, 2011 — The American College of Cardiology Foundation (ACCF) and the American Heart Association (AHA) ...

There have been several key news items from the U.S. stent market over the past year. These include the introduction of ...
September 20, 2011 — Boston Scientific Corp. has launched its Coyote Balloon Catheter, a highly deliverable and ultra ...
September 15, 2011 — Very positive results were reported in the first account of a clinical study evaluating the ...
September 13, 2011 – An U.S. Food and Drug Administration’s (FDA) will discuss recommendations for Cook Medical Zilver ...
September 8, 2011 – Abbott announced the initiation of ABSORB BTK, an international clinical trial evaluating the safety ...
September 1, 2011 – Medrad Inc. has acquired Pathway Medical Technologies Inc. to strength its vascular interventional ...
August 29, 2011 — The Guidelines on Peripheral Artery Disease (PAD) are the first document produced by the European ...

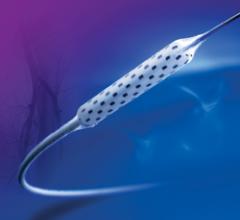
 November 14, 2011
November 14, 2011