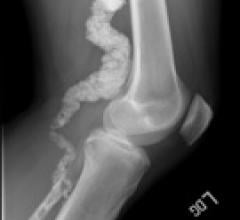
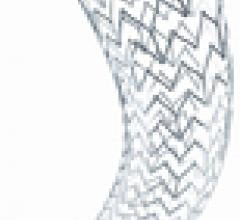
May 10, 2011 – Patients suffering from blockages of the arteries to the kidneys now have access to a new stenting ...
Peripheral Artery Disease (PAD)
This channel includes news, interventions, and new technology innovations for peripheral artery diease, PAD and critical limb ischemia.
April 13, 2011 – Patient enrollment has begun for the SuperNOVA clinical trial. It will examine the safety and ...
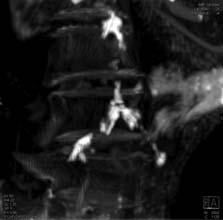
A U.S.-Egyptian research team has uncovered the earliest documented case of coronary atherosclerosis in a ...
April 4, 2011 – The U.S. Food and Drug Administration (FDA) has approved a 210-patient investigation devices ...
March 22, 2011 – The U.S. Food and Drug Administration (FDA) has approved a 210-patient investigation devices ...
March 18, 2011 – Circulating through the bloodstream of every human being is a rare and powerful type of cell, one ...
February 21, 2011 – The U.S. Food and Drug Administration has approved an endoprosthesis device for use on a lower ...
February 16, 2011 – To expand its portfolio of devices for lower extremity peripheral artery disease (PAD) ...
February 4, 2011 – Researchers at the National Institutes of Health's (NIH) Undiagnosed Diseases Program (UDP) ...
January 27, 2010 – The U.S. Food and Drug Administration (FDA) expanded the indication for the Bard Peripheral ...
January 24, 2011 – A new device for treating peripheral artery disease (PAD) is now available in Europe. W.L. Gore ...
January 20, 2011 – European CE mark approval was granted to expand the indication of Abbott’s Xience Prime ...
January 20, 2011 – Drug-coated stents hold promise as a safe and lasting solution for the treatment of clogged leg ...
January 18, 2011 – The first endovascular device approved for the treatment of pulmonary embolism (PE) was ...
January 13, 2011 – Enrollment has finished for the ORION trial designed to test the Epic self-expanding nitinol ...


 May 10, 2011
May 10, 2011