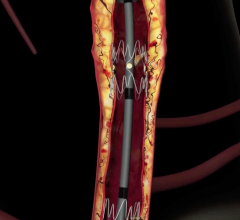
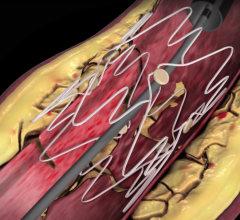
January 27, 2010 – The U.S. Food and Drug Administration (FDA) expanded the indication for the Bard Peripheral Vascular LifeStent and LifeStent XL nitinol self-expanding stents for the treatment of lesions up 240 mm in length.
The stents are now indicated are intended to improve luminal diameter in the treatment of symptomatic de-novo or restenotic lesions up to 240 mm in length in native superficial femoral artery (SFA) and/or proximal popliteal arteries with reference vessel diameters ranging from 4 to 6.5 mm.
The stents were originally cleared in February 2009 for the treatment of lesions up to 160 mm in length. The stents are equivalent in design with only one difference located at the crown section. The LifeStent stent has six tantalum radiopaque markers on both the distal and proximal ends of the stent, while the LifeStent XL stent does not have markers.
The expanded indication was granted in December 2010.
For more information: www.bardpv.com


 November 06, 2019
November 06, 2019