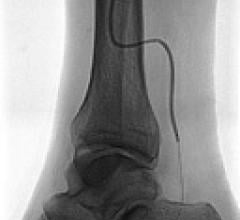
Drug-eluting balloons (DEBs) may offer new options in treating peripheral vessels and restenosis. DEBs already ...
Peripheral Artery Disease (PAD)
This channel includes news, interventions, and new technology innovations for peripheral artery diease, PAD and critical limb ischemia.

In recent years, many medical device companies saw the underserved lower extremity as an opportunity to enter the ...
April 22, 2010 – Broadening its product scope into the peripheral artery disease market, Medtronic today received ...
April 21, 2010 – More than 100,000 amputations are performed each year in the United States on patients with ...
March 11, 2010 – The FDA has cleared the first low-profile, premounted, balloon-expandable stent system for use ...

The general public usually does not take a big interest in cardiology or the interventional devices used to treat ...
March 2, 2010 — Leg arteries that become narrow decrease blood circulation and can cause these extremities to ...
March 2, 2010 — A new stent and delivery system for the treatment of peripheral artery disease (PAD) received CE ...
February 22, 2010 – As part of a comarketing and trademark license agreement between MEDRAD Inc. and B. Braun ...
February 11, 2010 — The New England Journal of Medicine (NEJM) published results showing the Bard FLAIR ...

A recently introduced atherectomy catheter offers a new class of device. It offers the advantages of two cutting ...
January 27, 2010 – New 6, 7 and 8 mm diameters of the Maris Plus self-expanding peripheral stent system, were ...
January 25, 2010 – Expanding its peripheral vascular disease product offerings, Medtronic Inc. today signed an ...
January 20, 2010 – The balloon material of the new REEF HP PTA Balloon Catheter was created for use in peripheral ...
January 13, 2010 – A study evaluating the effectiveness of the Clir-Path laser atherectomy system met its primary ...

 May 03, 2010
May 03, 2010