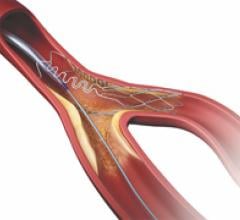
September 20, 2010 – The U.S. Food and Drug Administration (FDA) has approved the Integrity coronary stent system ...
Stents Bare Metal
Bare metal stents are mesh-like tubes of thin wire without a coating or covering. Ideally, bare metal stents will be covered by a new layer of endothelial cells, sealing it into the vessel wall, within a few weeks after implant. However, a percentage of patients have their body negatively respond to the implanted foreign body, resulting in the formation of neointimal hyperplasia, a growth of scar tissue over the stent. This can cause restenosis of the vessel segment, which led to the development of drug-eluting stents (DES) now used in most patients. However, DES require the patient to take long-term antiplatelet medication, so patients who are likely to be noncompliant are often given bare metal stents.
September 20, 2010 - Interim six-month results for a stent system designed to treat bifurcation lesions will be ...
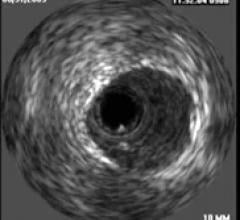
September 8. 2010 – Intravascular ultrasound (IVUS)-guided bifurcation stenting significantly reduced the four ...

In the world of endovascular devices, the rule is no longer one size fits all. The recent increase in the variety ...
August 19, 2010 – The Cardiovascular Research Foundation recently announced the late-breaking trials and first ...

There is no consensus about whether atherosclerotic renal artery stenosis should be stented or treated medically. There ...
May 12, 2010 - Increased detection and treatment of abdominal aortic aneurysms (AAAs) and peripheral arterial ...
May 6, 2010 – Patients who can postpone noncardiac surgery for at least six weeks after receiving a coronary ...
April 21, 2010 – A stent that captures endothelial progenitor cells is now combined with thin cobalt chromium ...
April 13, 2010 – More than 300 patients in Europe have been enrolled in a real-world study to assess the ...
March 30, 2010 – A new coronary stent recently launched in Europe is designed to gain easier access to distal ...
March 8, 2010 – Six-month follow-up data on the Stentys drug-eluting and bare metal stents showed a 4 percent ...
February 22, 2010 – During the Joint Interventional Meeting (JIM) Feb. 11-13 in Rome, the Cappella Sideguard 3.25 ...
December 8, 2009 – The first live U.K. cases involving Cappella’s self-expanding Sideguard Coronary Sidebranch ...
November 3, 2009 – The FDA said Boston Scientific recently changed the name of the company's Liberté Bare-Metal ...


 September 20, 2010
September 20, 2010